Nuclear pore complex: Difference between revisions
CSV import |
CSV import Tag: Removed redirect |
||
| Line 1: | Line 1: | ||
{{DISPLAYTITLE:Nuclear Pore Complex}} | |||
== | |||
The '''nuclear pore complex''' (NPC) is a large protein complex that spans the nuclear envelope, which is the double membrane surrounding the [[cell nucleus]]. It regulates the transport of molecules between the nucleus and the [[cytoplasm]], playing a crucial role in maintaining cellular function. | |||
File:Rancycle_nuclearimport_nuclearexport.png| | |||
File:3D-SIM-1_NPC_Confocal_vs_3D-SIM_detail.jpg| | ==Structure== | ||
File:Blausen_0212_CellNucleus.png| | [[File:Diagram_human_cell_nucleus.svg|Diagram of a human cell nucleus|thumb|right]] | ||
The nuclear pore complex is composed of multiple copies of approximately 30 different proteins known as nucleoporins. These nucleoporins assemble into a structure with an eightfold rotational symmetry, forming a central channel that allows the passage of ions, small molecules, and macromolecules. | |||
The NPC is organized into three main regions: | |||
* '''Cytoplasmic Ring''': Located on the cytoplasmic side of the nuclear envelope, this ring is involved in the initial recognition and binding of transport substrates. | |||
* '''Central Transport Channel''': This is the core of the NPC, where selective transport occurs. It is lined with phenylalanine-glycine (FG) repeat domains that facilitate the movement of transport receptors and their cargo. | |||
* '''Nuclear Ring''': Situated on the nuclear side, this ring anchors the NPC to the nuclear envelope and interacts with the nuclear lamina. | |||
==Function== | |||
[[File:NuclearPore_crop.png|Structure of a nuclear pore complex|thumb|left]] | |||
The primary function of the nuclear pore complex is to regulate the bidirectional transport of molecules across the nuclear envelope. This includes the import of proteins, such as [[transcription factors]] and [[histones]], and the export of [[RNA]] molecules and ribosomal subunits. | |||
Transport through the NPC is mediated by transport receptors known as karyopherins, which include importins and exportins. These receptors recognize nuclear localization signals (NLS) or nuclear export signals (NES) on their cargo molecules, facilitating their passage through the NPC. | |||
==Transport Mechanism== | |||
[[File:Rancycle_nuclearimport_nuclearexport.png|Diagram of nuclear import and export cycle|thumb|right]] | |||
The transport mechanism involves several steps: | |||
1. '''Recognition''': Cargo molecules with NLS or NES are recognized by importins or exportins, respectively. | |||
2. '''Translocation''': The receptor-cargo complex translocates through the NPC via interactions with FG-repeat domains. | |||
3. '''Release''': Once inside the nucleus or cytoplasm, the cargo is released, often facilitated by the small GTPase [[Ran]], which provides directionality to the transport process. | |||
==Role in Cellular Function== | |||
The NPC is essential for numerous cellular processes, including gene expression regulation, [[DNA replication]], and [[cell cycle]] control. By controlling the flow of information between the nucleus and cytoplasm, the NPC ensures that the cell responds appropriately to internal and external signals. | |||
==Pathology== | |||
Dysfunction of the nuclear pore complex is associated with various diseases, including [[cancer]], [[neurodegenerative diseases]], and [[viral infections]]. Alterations in nucleoporin expression or mutations can disrupt normal NPC function, leading to impaired cellular homeostasis. | |||
==Advanced Imaging Techniques== | |||
[[File:3D-SIM-1_NPC_Confocal_vs_3D-SIM_detail.jpg|Comparison of NPC imaging techniques|thumb|left]] | |||
Recent advances in imaging techniques, such as super-resolution microscopy, have provided detailed insights into the structure and dynamics of the NPC. These techniques allow for the visualization of individual nucleoporins and their interactions within the complex. | |||
==Related Pages== | |||
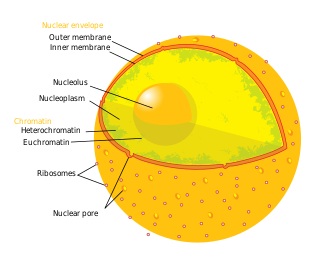
* [[Nuclear envelope]] | |||
* [[Nucleoplasm]] | |||
* [[Nucleolus]] | |||
* [[Chromatin]] | |||
[[File:Blausen_0212_CellNucleus.png|Illustration of a cell nucleus|thumb|right]] | |||
[[Category:Cell biology]] | |||
[[Category:Molecular biology]] | |||
[[Category:Protein complexes]] | |||
Latest revision as of 21:23, 4 March 2025
The nuclear pore complex (NPC) is a large protein complex that spans the nuclear envelope, which is the double membrane surrounding the cell nucleus. It regulates the transport of molecules between the nucleus and the cytoplasm, playing a crucial role in maintaining cellular function.
Structure[edit]
The nuclear pore complex is composed of multiple copies of approximately 30 different proteins known as nucleoporins. These nucleoporins assemble into a structure with an eightfold rotational symmetry, forming a central channel that allows the passage of ions, small molecules, and macromolecules.
The NPC is organized into three main regions:
- Cytoplasmic Ring: Located on the cytoplasmic side of the nuclear envelope, this ring is involved in the initial recognition and binding of transport substrates.
- Central Transport Channel: This is the core of the NPC, where selective transport occurs. It is lined with phenylalanine-glycine (FG) repeat domains that facilitate the movement of transport receptors and their cargo.
- Nuclear Ring: Situated on the nuclear side, this ring anchors the NPC to the nuclear envelope and interacts with the nuclear lamina.
Function[edit]

The primary function of the nuclear pore complex is to regulate the bidirectional transport of molecules across the nuclear envelope. This includes the import of proteins, such as transcription factors and histones, and the export of RNA molecules and ribosomal subunits.
Transport through the NPC is mediated by transport receptors known as karyopherins, which include importins and exportins. These receptors recognize nuclear localization signals (NLS) or nuclear export signals (NES) on their cargo molecules, facilitating their passage through the NPC.
Transport Mechanism[edit]

The transport mechanism involves several steps:
1. Recognition: Cargo molecules with NLS or NES are recognized by importins or exportins, respectively. 2. Translocation: The receptor-cargo complex translocates through the NPC via interactions with FG-repeat domains. 3. Release: Once inside the nucleus or cytoplasm, the cargo is released, often facilitated by the small GTPase Ran, which provides directionality to the transport process.
Role in Cellular Function[edit]
The NPC is essential for numerous cellular processes, including gene expression regulation, DNA replication, and cell cycle control. By controlling the flow of information between the nucleus and cytoplasm, the NPC ensures that the cell responds appropriately to internal and external signals.
Pathology[edit]
Dysfunction of the nuclear pore complex is associated with various diseases, including cancer, neurodegenerative diseases, and viral infections. Alterations in nucleoporin expression or mutations can disrupt normal NPC function, leading to impaired cellular homeostasis.
Advanced Imaging Techniques[edit]

Recent advances in imaging techniques, such as super-resolution microscopy, have provided detailed insights into the structure and dynamics of the NPC. These techniques allow for the visualization of individual nucleoporins and their interactions within the complex.
Related Pages[edit]
