2-Bromopropane: Difference between revisions
CSV import |
CSV import |
||
| Line 43: | Line 43: | ||
[[Category:Solvents]] | [[Category:Solvents]] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
<gallery> | |||
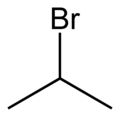
File:2-bromopropane-2D-skeletal.png|2-Bromopropane skeletal structure | |||
File:2-bromopropane-2D-flat.png|2-Bromopropane flat structure | |||
</gallery> | |||
Latest revision as of 01:04, 18 February 2025
2-Bromopropane[edit]


2-Bromopropane, also known as isopropyl bromide, is an organobromine compound with the chemical formula C_H_Br. It is a colorless liquid that is used in various industrial applications, including as a solvent and an intermediate in organic synthesis.
Structure and Properties[edit]
2-Bromopropane is a haloalkane with a molecular structure consisting of a three-carbon alkane chain with a bromine atom attached to the second carbon. This structure gives it the IUPAC name 2-bromopropane. The presence of the bromine atom makes it a polar molecule, which influences its physical and chemical properties.
Physical Properties[edit]
2-Bromopropane is a colorless liquid at room temperature. It has a boiling point of approximately 59 °C and a density of about 1.31 g/cm_. It is slightly soluble in water but miscible with most organic solvents.
Chemical Properties[edit]
As a haloalkane, 2-bromopropane is reactive in nucleophilic substitution reactions. It can undergo SN1 and SN2 reactions, depending on the conditions and the nucleophile involved. It is also susceptible to elimination reactions, which can lead to the formation of alkenes.
Synthesis[edit]
2-Bromopropane can be synthesized through the halogenation of propane using bromine in the presence of light or heat. Alternatively, it can be prepared by the reaction of isopropanol with hydrogen bromide in the presence of a strong acid catalyst.
Uses[edit]
2-Bromopropane is used in various industrial applications:
- As a solvent in the manufacturing of pharmaceuticals and agrochemicals.
- As an intermediate in the synthesis of other organic compounds.
- In the production of flame retardants and plasticizers.
Health and Safety[edit]
Exposure to 2-bromopropane can pose health risks. It is important to handle it with care, using appropriate personal protective equipment and following safety guidelines. Prolonged exposure can lead to respiratory and dermatological issues.
Related Pages[edit]
Gallery[edit]
-
2D skeletal structure of 2-Bromopropane
-
2D flat structure of 2-Bromopropane
-
2-Bromopropane skeletal structure
-
2-Bromopropane flat structure