Tetraethyl pyrophosphate: Difference between revisions
CSV import |
CSV import |
||
| Line 49: | Line 49: | ||
{{medicine-stub}} | {{medicine-stub}} | ||
<gallery> | |||
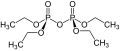
File:Tetraethyl_pyrophosphate_Structural_Formula_V2.svg|Structural formula of Tetraethyl pyrophosphate | |||
</gallery> | |||
Latest revision as of 01:49, 17 February 2025
Tetraethyl pyrophosphate (TEPP) is an organophosphate compound with the chemical formula (C₂H₅O)₄P₂O₇. It is a colorless, viscous liquid that is highly toxic and was historically used as an insecticide.
Chemical Structure and Properties[edit]
Tetraethyl pyrophosphate consists of two phosphorus atoms connected by an oxygen bridge, with each phosphorus atom bonded to two ethoxy groups. The chemical structure can be represented as:
(C₂H₅O)₂P-O-P(O)(OC₂H₅)₂
TEPP is highly soluble in organic solvents and hydrolyzes rapidly in water, forming phosphoric acid and ethanol.
Synthesis[edit]
TEPP can be synthesized by the reaction of phosphorus oxychloride (POCl₃) with ethanol in the presence of a base. The reaction proceeds as follows:
POCl₃ + 4 C₂H₅OH → (C₂H₅O)₄P₂O₇ + 3 HCl
Uses[edit]
Historically, TEPP was used as an insecticide due to its potent acetylcholinesterase inhibition properties. It was effective against a wide range of insects but has largely been replaced by less toxic and more stable compounds.
Toxicity[edit]
TEPP is highly toxic to humans and other mammals. It acts as an acetylcholinesterase inhibitor, leading to the accumulation of acetylcholine in the nervous system, which can cause muscle paralysis and respiratory failure. Symptoms of TEPP poisoning include headache, dizziness, sweating, salivation, nausea, and convulsions.
Safety and Handling[edit]
Due to its high toxicity, TEPP must be handled with extreme care. Protective clothing, gloves, and eye protection are essential when working with this compound. In case of exposure, immediate medical attention is required.
Environmental Impact[edit]
TEPP is highly toxic to aquatic life and can cause long-term adverse effects in the aquatic environment. Its use has been restricted or banned in many countries due to its environmental and health hazards.
Related Compounds[edit]
See Also[edit]
References[edit]
<references group="" responsive="1"></references>
External Links[edit]
-
Structural formula of Tetraethyl pyrophosphate