Galantamine total synthesis: Difference between revisions
CSV import |
CSV import |
||
| Line 32: | Line 32: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
{{Pharmacology-stub}} | {{Pharmacology-stub}} | ||
<gallery> | |||
File:Galantamine.svg|Galantamine structure | |||
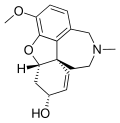
File:GalanthamineNumberingAndStereocenters.png|Galanthamine numbering and stereocenters | |||
File:GalanthamineTotalResolution.png|Galanthamine total resolution | |||
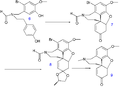
File:NarwedineSynthesisPartA.png|Narwedine synthesis part A | |||
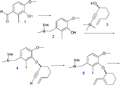
File:NarwedineSynthesisPartB.png|Narwedine synthesis part B | |||
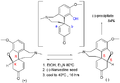
File:GalanthamineIndustrial.png|Galanthamine industrial synthesis | |||
File:Galanthamine_Trost_2005.svg|Galanthamine Trost synthesis 2005 | |||
File:GalanthamineTotalSynthesis2007A.png|Galanthamine total synthesis 2007 part A | |||
File:GalanthamineTotalSynthesis2007B.png|Galanthamine total synthesis 2007 part B | |||
</gallery> | |||
Latest revision as of 12:25, 18 February 2025
Galantamine Total Synthesis refers to the chemical process of synthesizing Galantamine, a tertiary alkaloid used primarily for the management of mild to moderate Alzheimer's disease. Galantamine, naturally derived from flowers such as those of the Galanthus (snowdrop) and Narcissus (daffodil) species, has garnered significant interest for its therapeutic potential, leading to the development of various synthetic routes to produce this compound in the laboratory.
History[edit]
The quest for the total synthesis of Galantamine began shortly after its isolation and the elucidation of its structure. The complexity of its molecular structure, characterized by a fused four-ring system with multiple chiral centers, posed a significant challenge for synthetic chemists. Early attempts focused on the construction of the core skeleton, with subsequent efforts improving yield, stereoselectivity, and overall efficiency.
Synthetic Approaches[edit]
Several synthetic approaches have been developed for the total synthesis of Galantamine, each with its own advantages and challenges. These methods generally involve the construction of the bicyclic core, followed by the introduction of the tertiary amine and the final functional groups.
Biomimetic Synthesis[edit]
Biomimetic synthesis attempts to mimic the natural biosynthetic pathway of Galantamine in plants. This approach often involves the cyclization of a suitably substituted precursor to form the core structure, followed by enzymatic resolution to achieve the desired stereochemistry.
Chemoenzymatic Synthesis[edit]
Chemoenzymatic synthesis combines chemical synthesis with enzymatic methods to introduce chirality and complexity. This method typically involves the use of enzymes to resolve racemic mixtures or to perform selective modifications on a synthetic intermediate.
Total Chemical Synthesis[edit]
Total chemical synthesis relies entirely on chemical reactions to construct the Galantamine molecule from simple starting materials. This approach allows for greater control over the stereochemistry and enables the introduction of modifications that may enhance the compound's pharmacological properties.
Key Challenges[edit]
The total synthesis of Galantamine presents several challenges, including the efficient construction of its complex molecular architecture, the control of stereochemistry at multiple chiral centers, and the development of scalable and economically viable synthetic routes.
Clinical Significance[edit]
Synthetic Galantamine provides a valuable alternative to the extraction from natural sources, ensuring a consistent and reliable supply of this important therapeutic agent. Its role in the treatment of Alzheimer's disease, through the modulation of cholinergic function, highlights the importance of synthetic efforts in providing access to this pharmacologically significant molecule.
Future Directions[edit]
Research into the total synthesis of Galantamine continues to evolve, with efforts focusing on improving efficiency, reducing environmental impact, and exploring novel synthetic pathways. The development of enantioselective and catalytic methods remains a key area of interest, promising to enhance the accessibility and sustainability of Galantamine synthesis.
-
Galantamine structure
-
Galanthamine numbering and stereocenters
-
Galanthamine total resolution
-
Narwedine synthesis part A
-
Narwedine synthesis part B
-
Galanthamine industrial synthesis
-
Galanthamine Trost synthesis 2005
-
Galanthamine total synthesis 2007 part A
-
Galanthamine total synthesis 2007 part B