Longifolene: Difference between revisions
CSV import |
CSV import |
||
| Line 28: | Line 28: | ||
{{pharmacology-stub}} | {{pharmacology-stub}} | ||
== Longifolene == | |||
<gallery> | |||
File:Longifolene_plus_acsv.svg|Longifolene | |||
File:Longifolene_Biosynthesis_Mechanism.png|Longifolene Biosynthesis Mechanism | |||
File:Longifolene_total_synthesis_by_Corey.svg|Longifolene Total Synthesis by Corey | |||
</gallery> | |||
Latest revision as of 03:55, 18 February 2025
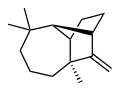
Longifolene is a naturally occurring, oily liquid hydrocarbon found primarily in the high-boiling fraction of certain pine resins. The name is derived from that of a pine species from which it was first isolated, Pinus longifolia. It is chiral and thus can exist in enantiomeric forms. However, it is usually encountered as a racemic mixture.
Chemical Structure and Properties[edit]
Longifolene is a tricyclic sesquiterpene. This means it is composed of three five-membered rings and is derived from the primary building block of terpenes, isoprene. Its molecular formula is C15H24. It is a colorless to pale yellow liquid with a woody and earthy aroma, reminiscent of freshly cut wood and soil.
Occurrence and Extraction[edit]
Longifolene is found in certain species of pines, particularly in the high-boiling fraction of their resins. It can be extracted using steam distillation or solvent extraction. The yield of longifolene from these processes is typically low, and it is often accompanied by other sesquiterpenes.
Uses[edit]
Longifolene is used primarily as a starting material in the manufacture of certain perfumes and aromatic chemicals. Its woody and earthy aroma makes it a valuable ingredient in the creation of certain types of fragrances. It is also used in the synthesis of vitamin E and in the manufacture of certain types of plastics.
Synthesis[edit]
The synthesis of longifolene has been a subject of interest for organic chemists. The first total synthesis was reported in 1964 by the group of Robert Burns Woodward. The Woodward longifolene synthesis involves the use of a double Wittig reaction, a powerful tool in organic synthesis.
Safety[edit]
As with any chemical, proper handling and use of longifolene are essential. It is recommended to use personal protective equipment (PPE) when handling longifolene. It is also advisable to use it in a well-ventilated area to avoid inhalation of its vapors.
See Also[edit]
Longifolene[edit]
-
Longifolene
-
Longifolene Biosynthesis Mechanism
-
Longifolene Total Synthesis by Corey