Solvation: Difference between revisions
CSV import |
CSV import |
||
| Line 32: | Line 32: | ||
{{stub}} | {{stub}} | ||
<gallery> | |||
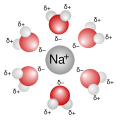
File:Na+H2O.svg|Sodium ion solvated by water molecules | |||
File:Nile_red_01.jpg|Nile red dye in solution | |||
File:Effect_of_solvent_on_solubility.png|Effect of solvent on solubility | |||
</gallery> | |||
Latest revision as of 01:32, 18 February 2025
Solvation refers to the interaction of solvent with dissolved molecules. Both ionic and molecular solutes can be solvated. This interaction is of a chemical nature and is key in understanding many chemical processes.
Overview[edit]
Solvation involves different types of force, including dispersion, electrostatic, and hydrogen bonding. Ion-dipole and dipole-dipole interactions are also involved.
The solvation process is often dynamic, with solute molecules or ions moving from one solvation site to another. This is particularly true in protic solvents, where hydrogen bonds are continually forming and breaking.
Solvation and Solubility[edit]
The solvation process plays a significant role in solubility, partitioning, and reactions in solution. The solubility of a solute in a particular solvent is determined by the balance between the energy of solvation and the energy required to separate the solute into its constituent particles.
Solvation Models[edit]
Several models have been proposed to quantify solvation effects, including the Born model, the Langevin dipole model, and the Lippert-Mataga model. These models aim to explain the behavior of solutes in solution and predict their properties.
See Also[edit]
References[edit]
<references />