Palladium: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import Tags: mobile edit mobile web edit |
||
| Line 42: | Line 42: | ||
{{stub}} | {{stub}} | ||
{{dictionary-stub1}} | {{dictionary-stub1}} | ||
<gallery> | |||
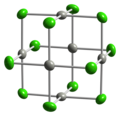
File:Alpha-palladium(II)-chloride-xtal-3D-balls.png|Alpha-palladium(II) chloride crystal 3D balls | |||
File:Pd6Cl12-from-xtal-1996-CM-3D-ellipsoids.png|Pd6Cl12 from crystal 1996 CM 3D ellipsoids | |||
File:Pd(OAc)2.jpg|Palladium(II) acetate | |||
File:Platinum-palladium_ore,_Stillwater_mine_MT.JPG|Platinum-palladium ore, Stillwater mine MT | |||
File:Sulfidic_serpentintite_(platinum-palladium_ore)_Johns-Manville_Reef,_Stillwater_Complex.jpg|Sulfidic serpentinite (platinum-palladium ore) Johns-Manville Reef, Stillwater Complex | |||
File:2005palladium_(mined).PNG|2005 palladium (mined) | |||
File:Aufgeschnittener_Metall_Katalysator_für_ein_Auto.jpg|Cut open metal catalyst for a car | |||
File:25_rubles_palladium_1989_Ivan_III.jpg|25 rubles palladium 1989 Ivan III | |||
File:Kumada_Catalytic_Cycle.png|Kumada Catalytic Cycle | |||
File:Wollaston_William_Hyde_Jackson_color.jpg|William Hyde Wollaston | |||
File:Potw1749a_Pallas_crop.png|Pallas | |||
</gallery> | |||
Latest revision as of 21:23, 23 February 2025
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas.
Characteristics[edit]
Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). These have similar chemical properties, but palladium has the lowest melting point and is the least dense of them.
Applications[edit]
The largest use of palladium today is in catalytic converters. Palladium is also used in electronics, dentistry, medicine, hydrogen purification, chemical applications, groundwater treatment, and jewelry. Palladium is a key component of fuel cells, which react hydrogen with oxygen to produce electricity, heat, and water.
Occurrence[edit]
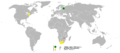
Palladium is found in the Earth's crust at a concentration of 0.015 ppm. It occurs naturally in native form and alloyed with gold and other platinum-group metals in placer deposits of the Ural Mountains, Australia, Ethiopia, and the Americas.
History[edit]
Palladium was discovered in 1803, in London, by English chemist William Hyde Wollaston. He examined the residues left from dissolving the platinum ore in aqua regia, a mixture of hydrochloric and nitric acids.
Health effects[edit]
Palladium compounds are considered to be of low toxicity compared to other heavy metals, and their primary health effect is allergic reactions.
See also[edit]
References[edit]
<references />
External links[edit]
- WebElements.com – Palladium, includes historical prices
- Palladium at The Periodic Table of Videos (University of Nottingham)
|
|
|
-
Alpha-palladium(II) chloride crystal 3D balls
-
Pd6Cl12 from crystal 1996 CM 3D ellipsoids
-
Palladium(II) acetate
-
Platinum-palladium ore, Stillwater mine MT
-
Sulfidic serpentinite (platinum-palladium ore) Johns-Manville Reef, Stillwater Complex
-
2005 palladium (mined)
-
Cut open metal catalyst for a car
-
25 rubles palladium 1989 Ivan III
-
Kumada Catalytic Cycle
-
William Hyde Wollaston
-
Pallas