Molybdenum hexafluoride: Difference between revisions
CSV import |
No edit summary |
||
| Line 21: | Line 21: | ||
[[Category:Fluorides]] | [[Category:Fluorides]] | ||
{{chem-stub}} | {{chem-stub}} | ||
== Molybdenum hexafluoride gallery == | == Molybdenum hexafluoride gallery == | ||
<gallery> | <gallery> | ||
Latest revision as of 00:41, 17 March 2025
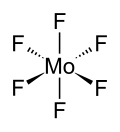
Molybdenum hexafluoride', also known by its chemical formula MoF6, is a compound consisting of one molybdenum atom and six fluorine atoms. This chemical compound is significant in the field of chemistry, particularly in inorganic chemistry, due to its unique properties and applications. Molybdenum hexafluoride is a colorless, volatile, and highly reactive gas under standard conditions of temperature and pressure.
Properties[edit]
Molybdenum hexafluoride is known for its high reactivity and volatility. It is one of the few substances that can directly react with glass, which is why it is stored in metal containers. It has a melting point of 17.5°C and a boiling point of 34°C, which makes it a gas at room temperature but easily liquefiable under pressure or cooling. In terms of its chemical behavior, MoF6 is a strong oxidizing agent and can react violently with organic materials and water.
Synthesis[edit]
The synthesis of molybdenum hexafluoride typically involves the direct reaction of molybdenum metal with elemental fluorine. This process requires strict control of conditions to ensure safety and yield efficiency:
\[ \text{Mo} + 3\text{F}_2 \rightarrow \text{MoF}_6 \]
This reaction is highly exothermic and must be conducted in a controlled environment to prevent accidents due to the compound's reactive nature.
Applications[edit]
Molybdenum hexafluoride is primarily used in the nuclear industry for the isotope separation of molybdenum isotopes. This is crucial for the production of molybdenum-99, a parent isotope of technetium-99m, which is widely used in medical diagnostics. Additionally, MoF6 serves as a precursor in the preparation of molybdenum metal and its compounds, which have various industrial applications due to their high strength and resistance to corrosion.
Safety[edit]
Handling molybdenum hexafluoride requires strict safety measures due to its corrosive nature and reactivity with water, which can release toxic hydrogen fluoride gas. Proper protective equipment, such as gloves and goggles, and the use of corrosion-resistant containers are essential when working with this compound.
Molybdenum hexafluoride gallery[edit]
-
Molybdenum(VI) fluoride
-
Molybdenum hexafluoride from crystal 3D