Hydrogenation: Difference between revisions
CSV import |
No edit summary |
||
| Line 1: | Line 1: | ||
[[File:Hydrogenation_on_catalyst.svg|Hydrogenation|thumb]] | |||
[[File:Crabtree.svg|Hydrogenation|thumb|left]] | |||
[[File:Cyclooctadiene-rhodium-chloride-dimer-2D-skeletal.png|Hydrogenation|thumb]] | |||
[[File:(S)-iPr-PHOX.svg|Hydrogenation|thumb|left]] | |||
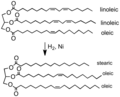
'''Hydrogenation''' is a chemical reaction that adds hydrogen ([[H2]]) to a molecule. This process is commonly used in the food industry to convert unsaturated fats to saturated fats, which are more stable and have a longer shelf life. | '''Hydrogenation''' is a chemical reaction that adds hydrogen ([[H2]]) to a molecule. This process is commonly used in the food industry to convert unsaturated fats to saturated fats, which are more stable and have a longer shelf life. | ||
| Line 11: | Line 15: | ||
== Health effects == | == Health effects == | ||
The consumption of hydrogenated fats has been linked to an increased risk of [[heart disease]]. This is because the process of hydrogenation creates [[trans fats]], which raise levels of low-density lipoprotein (LDL, or "bad" cholesterol) and lower levels of high-density lipoprotein (HDL, or "good" cholesterol) in the blood. | The consumption of hydrogenated fats has been linked to an increased risk of [[heart disease]]. This is because the process of hydrogenation creates [[trans fats]], which raise levels of low-density lipoprotein (LDL, or "bad" cholesterol) and lower levels of high-density lipoprotein (HDL, or "good" cholesterol) in the blood. | ||
== Gallery == | |||
<gallery> | |||
File:Dichlorotris(triphenylphosphine)ruthenium(II).png|Hydrogenation | |||
File:CarvoneH2.png|Hydrogenation | |||
File:PhC2HH2.png|Hydrogenation | |||
File:ImineH2.png|Hydrogenation | |||
File:ResorcinolH2.png|Hydrogenation | |||
File:SuccPdH2.png|Hydrogenation | |||
File:THintermed.png|Hydrogenation | |||
File:H2forMargerin.png|Hydrogenation | |||
</gallery> | |||
== See also == | == See also == | ||
| Line 24: | Line 40: | ||
[[Category:Industrial processes]] | [[Category:Industrial processes]] | ||
{{stub}} | {{stub}} | ||
Latest revision as of 15:04, 9 March 2025




Hydrogenation is a chemical reaction that adds hydrogen (H2) to a molecule. This process is commonly used in the food industry to convert unsaturated fats to saturated fats, which are more stable and have a longer shelf life.
Process[edit]
Hydrogenation is carried out by reacting the molecule with hydrogen gas in the presence of a catalyst. The catalyst is usually a metal such as nickel, palladium, or platinum. The reaction takes place at high temperatures and pressures.
Uses[edit]
Hydrogenation is widely used in the food industry to convert unsaturated fats to saturated fats. This process increases the stability and shelf life of the fats. It is also used in the production of margarine and shortening.
In the chemical industry, hydrogenation is used to convert alkenes to alkanes and to reduce carbonyl groups to alcohols. It is also used in the production of ammonia through the Haber process.
Health effects[edit]
The consumption of hydrogenated fats has been linked to an increased risk of heart disease. This is because the process of hydrogenation creates trans fats, which raise levels of low-density lipoprotein (LDL, or "bad" cholesterol) and lower levels of high-density lipoprotein (HDL, or "good" cholesterol) in the blood.
Gallery[edit]
-
Hydrogenation
-
Hydrogenation
-
Hydrogenation
-
Hydrogenation
-
Hydrogenation
-
Hydrogenation
-
Hydrogenation
-
Hydrogenation
See also[edit]
References[edit]
<references />