Acid dissociation constant: Difference between revisions
CSV import |
CSV import |
||
| Line 75: | Line 75: | ||
[[Category:Acid-base chemistry]] | [[Category:Acid-base chemistry]] | ||
<gallery> | |||
File:Acetic-acid-dissociation-3D-balls.png|Acid dissociation constant | |||
File:PK_acetic_acid.png|Acid dissociation constant | |||
File:Weak_acid_speciation.svg|Acid dissociation constant | |||
File:H3PO4_speciation.png|Acid dissociation constant | |||
File:Citric_acid_speciation.png|Acid dissociation constant | |||
File:Carboxylic_acid_dimers.png|Acid dissociation constant | |||
File:Acetic_acid_pK_dioxane_water.png|Acid dissociation constant | |||
File:Chloroacetic_pka.png|Acid dissociation constant | |||
File:Fumaric-acid-2D-skeletal.png|Acid dissociation constant | |||
File:Maleic-acid-2D-skeletal-A.svg|Acid dissociation constant | |||
File:Proton_sponge.svg|Acid dissociation constant | |||
File:Oxalic_acid_titration_grid.png|Acid dissociation constant | |||
</gallery> | |||
Latest revision as of 11:51, 18 February 2025
Acid Dissociation Constant[edit]
The acid dissociation constant, denoted as Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions. The equilibrium is represented as:
- HA _ H+ + A-
where HA is a generic acid that dissociates into a proton (H+) and its conjugate base (A-). The larger the value of Ka, the more the acid dissociates, and the stronger the acid.
Definition and Expression[edit]
The acid dissociation constant is defined by the equation:
- Ka = \( \frac{[\text{H}^+][\text{A}^-]}{[\text{HA}]} \)
where [H+], [A-], and [HA] are the molar concentrations of the hydrogen ion, the conjugate base, and the undissociated acid, respectively.
pKa[edit]
The pKa is the negative logarithm (base 10) of the acid dissociation constant:
- pKa = -log10Ka
A lower pKa value indicates a stronger acid, which means it more fully dissociates in solution.
Factors Affecting Acid Strength[edit]
Several factors influence the strength of an acid, including:
- Electronegativity: More electronegative atoms can stabilize the negative charge on the conjugate base, increasing acid strength.
- Resonance: Delocalization of charge through resonance can stabilize the conjugate base.
- Inductive Effect: Electron-withdrawing groups can stabilize the conjugate base through the inductive effect.
- Hybridization: The s-character of the hybrid orbitals can affect acidity; more s-character can lead to stronger acids.
Examples of Acid Dissociation Constants[edit]
Acetic Acid[edit]
Acetic acid (CH3COOH) is a weak acid with a pKa of approximately 4.76. It partially dissociates in water to form acetate ions (CH3COO-) and hydrogen ions (H+).
Phosphoric Acid[edit]

Phosphoric acid (H3PO4) is a triprotic acid with three dissociation constants, corresponding to the loss of each proton.
Citric Acid[edit]
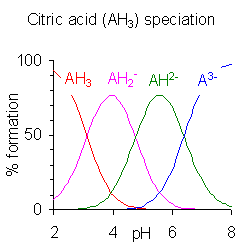
Citric acid (C6H8O7) is a weak organic acid with three carboxyl groups, each with its own dissociation constant.
Related Concepts[edit]
Related Pages[edit]
Gallery[edit]
-
pKa of acetic acid
-
Weak acid speciation
-
Carboxylic acid dimers
-
Acetic acid pKa in dioxane-water
-
Chloroacetic acid pKa
-
Fumaric acid structure
-
Maleic acid structure
-
Proton sponge
-
Acid dissociation constant
-
Acid dissociation constant
-
Acid dissociation constant
-
Acid dissociation constant
-
Acid dissociation constant
-
Acid dissociation constant
-
Acid dissociation constant
-
Acid dissociation constant
-
Acid dissociation constant
-
Acid dissociation constant
-
Acid dissociation constant
-
Acid dissociation constant









