Ubiquitin-activating enzyme: Difference between revisions
CSV import |
CSV import |
||
| Line 38: | Line 38: | ||
[[Category:Enzymes]] | [[Category:Enzymes]] | ||
[[Category:Ubiquitin system]] | [[Category:Ubiquitin system]] | ||
<gallery caption="Ubiquitin-activating_enzyme"> | |||
File:Ubiquitin-activating_enzyme_bound_to_Ubiquitin.png|Ubiquitin-activating enzyme bound to Ubiquitin | |||
File:Ubiquitin-activating_enzyme_bound_to_ATP_and_ubiquitin_substrate.png|Ubiquitin-activating enzyme bound to ATP and ubiquitin substrate | |||
File:Ubiquitin_activating_enzyme_cysteine_highlighted.png|Ubiquitin activating enzyme cysteine highlighted | |||
File:Cys_and_Arg_active_site_of_ubiquitin_activating_enzyme.png|Cys and Arg active site of ubiquitin activating enzyme | |||
File:Mechanism_for_the_binding_of_ATP_and_then_Ubiquitin_substrate_to_the_ubiquitin_activating_enzyme.png|Mechanism for the binding of ATP and then Ubiquitin substrate to the ubiquitin activating enzyme | |||
File:Ubiquitylation.png|Ubiquitylation | |||
</gallery> | |||
Latest revision as of 11:34, 18 February 2025
Enzyme that activates ubiquitin in the ubiquitination process
Ubiquitin-activating enzyme (E1) is a crucial enzyme in the process of ubiquitination, which is the attachment of ubiquitin to a substrate protein. This process is essential for various cellular functions, including protein degradation, cell cycle regulation, and DNA repair.
Structure[edit]
Ubiquitin-activating enzymes are characterized by their ability to bind ATP and ubiquitin. The enzyme has a catalytic cysteine residue that forms a thioester bond with ubiquitin. The structure of E1 is complex, involving multiple domains that facilitate its interaction with ATP and ubiquitin.

Mechanism[edit]
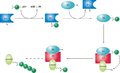
The ubiquitin-activating enzyme initiates the ubiquitination process by catalyzing the adenylation of ubiquitin, which involves the binding of ATP and ubiquitin to form a ubiquitin-adenylate intermediate. This is followed by the transfer of ubiquitin to the active site cysteine of E1, forming a thioester bond.

Function[edit]
E1 enzymes are responsible for the activation of ubiquitin, which is then transferred to a ubiquitin-conjugating enzyme (E2). This activation is the first step in the ubiquitination cascade, which ultimately leads to the attachment of ubiquitin to target proteins. This process is vital for the regulation of protein turnover and cellular homeostasis.
Active Site[edit]
The active site of the ubiquitin-activating enzyme contains a cysteine residue that is crucial for its catalytic activity. This cysteine forms a transient thioester bond with ubiquitin, facilitating its transfer to E2 enzymes.

Role in Ubiquitination[edit]
Ubiquitination is a post-translational modification that tags proteins for degradation by the proteasome. The ubiquitin-activating enzyme is the first enzyme in the ubiquitination pathway, playing a pivotal role in the regulation of protein levels within the cell.

Related pages[edit]
References[edit]
- Hershko, A., & Ciechanover, A. (1998). The ubiquitin system. Annual Review of Biochemistry, 67, 425-479.
- Pickart, C. M. (2001). Mechanisms underlying ubiquitination. Annual Review of Biochemistry, 70, 503-533.
- Ubiquitin-activating_enzyme
-
Ubiquitin-activating enzyme bound to Ubiquitin
-
Ubiquitin-activating enzyme bound to ATP and ubiquitin substrate
-
Ubiquitin activating enzyme cysteine highlighted
-
Cys and Arg active site of ubiquitin activating enzyme
-
Mechanism for the binding of ATP and then Ubiquitin substrate to the ubiquitin activating enzyme
-
Ubiquitylation