Perchloryl fluoride: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
No edit summary |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 31: | Line 31: | ||
[[Category:Oxidizing agents]] | [[Category:Oxidizing agents]] | ||
{{Chem-stub}} | {{Chem-stub}} | ||
==Perchloryl_fluoride== | |||
<gallery> | |||
File:Perchloryl-fluoride-2D.png|Perchloryl fluoride 2D structure | |||
File:Perchloryl-fluoride-3D-vdW.png|Perchloryl fluoride 3D van der Waals model | |||
</gallery> | |||
Latest revision as of 00:57, 17 March 2025
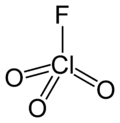
Perchloryl fluoride is a chemical compound with the formula ClO_3F. It is a colorless gas at room temperature and is highly reactive, as well as toxic. Perchloryl fluoride is used primarily in the synthesis of organic compounds and as an oxidizing agent in rocket propellants. Due to its reactive nature, it is of significant interest in both industrial and academic settings for its potential applications and hazards.
Properties[edit]
Perchloryl fluoride is characterized by its strong oxidizing properties. It is capable of supporting combustion, even in the absence of atmospheric oxygen, which makes it particularly useful in closed-system applications such as rocket propulsion. The compound is stable under standard conditions but decomposes upon contact with water, releasing hydrochloric acid (HCl) and oxygen gas (O2), which can pose significant handling risks.
Synthesis[edit]
The synthesis of perchloryl fluoride typically involves the reaction of chlorine trifluoride (ClF3) with sodium perchlorate (NaClO4). This process requires careful control of reaction conditions to prevent uncontrolled decomposition or reaction, which could lead to explosive outcomes.
Applications[edit]
Organic Synthesis[edit]
In organic chemistry, perchloryl fluoride is used as a fluorinating agent, introducing fluorine atoms into organic molecules. This can significantly alter the physical and chemical properties of the compounds, making them more reactive or altering their biological activity.
Rocket Propellants[edit]
Due to its strong oxidizing properties, perchloryl fluoride is used in some rocket propellant formulations. It can significantly increase the efficiency of the propellant, providing higher thrust compared to traditional oxidizers.
Safety and Handling[edit]
Handling perchloryl fluoride requires strict safety measures due to its toxic and reactive nature. Exposure to the compound can lead to severe respiratory issues and chemical burns. In industrial settings, appropriate personal protective equipment (PPE) and engineering controls are essential to mitigate these risks.
Environmental Impact[edit]
The environmental impact of perchloryl fluoride is a concern due to its potential to decompose into chlorine and oxygen radicals, which can contribute to ozone layer depletion. As such, its use and disposal are regulated under various international environmental protection guidelines.
See Also[edit]
Perchloryl_fluoride[edit]
-
Perchloryl fluoride 2D structure
-
Perchloryl fluoride 3D van der Waals model