Triatomic molecule: Difference between revisions
CSV import |
CSV import |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|A molecule composed of three atoms}} | |||
{{Use dmy dates|date=October 2023}} | |||
A '''triatomic molecule''' is a [[molecule]] composed of three [[atom]]s, which may or may not be of the same [[chemical element]]. Triatomic molecules can be [[linear molecule|linear]] or [[nonlinear molecule|nonlinear]] in shape. | |||
==Examples== | ==Examples== | ||
=== | ===Carbon dioxide=== | ||
[[File:Carbon-dioxide-3D-vdW.svg|thumb|right|200px|3D model of a carbon dioxide molecule]] | |||
[[Carbon dioxide]] (CO_) is a linear triatomic molecule consisting of one [[carbon]] atom and two [[oxygen]] atoms. It is a significant component of the Earth's atmosphere and is involved in the [[carbon cycle]]. Carbon dioxide is a greenhouse gas and plays a crucial role in [[photosynthesis]]. | |||
===Ozone | ===Ozone=== | ||
Ozone is a triatomic molecule | [[File:Ozone-elpot-3D-vdW.png|thumb|right|200px|3D model of an ozone molecule]] | ||
[[Ozone]] (O_) is a nonlinear triatomic molecule composed of three oxygen atoms. It is found in the Earth's [[stratosphere]] and forms the [[ozone layer]], which absorbs most of the Sun's harmful [[ultraviolet]] radiation. Ozone is also present at ground level as a pollutant. | |||
== | ===Trihydrogen cation=== | ||
The | [[File:Trihydrogen-cation-3D-vdW.png|thumb|right|200px|3D model of a trihydrogen cation]] | ||
The [[trihydrogen cation]] (H__) is a positively charged ion consisting of three hydrogen atoms. It is an important species in [[interstellar medium|interstellar space]] and plays a role in the chemistry of [[star formation]]. | |||
== | ==Properties== | ||
Triatomic molecules | Triatomic molecules can exhibit a variety of properties depending on their structure and composition. Linear triatomic molecules, such as carbon dioxide, have a straight-line geometry, while nonlinear molecules, like ozone, have a bent shape. These structural differences affect their [[vibrational mode|vibrational]] and [[rotational mode|rotational]] spectra. | ||
== | ==Applications== | ||
Triatomic molecules are important in various fields of science and technology. Carbon dioxide is used in [[carbonated beverage]]s, [[fire extinguisher]]s, and as a refrigerant. Ozone is used in [[water purification]] and [[air purification]] systems. The trihydrogen cation is studied in [[astrophysics]] and [[quantum chemistry]]. | |||
==Related pages== | |||
* [[Diatomic molecule]] | |||
* [[Polyatomic molecule]] | |||
* [[Molecular geometry]] | * [[Molecular geometry]] | ||
==References== | |||
* Atkins, P., & de Paula, J. (2006). ''Physical Chemistry''. Oxford University Press. | |||
* Herzberg, G. (1966). ''Molecular Spectra and Molecular Structure''. Van Nostrand Reinhold. | |||
[[Category:Molecules]] | |||
[[Category:Chemical bonding]] | |||
<gallery> | |||
File:Carbon-dioxide-3D-vdW.svg|Carbon dioxide molecule | |||
File:Ozone-elpot-3D-vdW.png|Ozone molecule | |||
File:Trihydrogen-cation-3D-vdW.png|Trihydrogen cation | |||
</gallery> | |||
Latest revision as of 02:14, 18 February 2025
A molecule composed of three atoms
A triatomic molecule is a molecule composed of three atoms, which may or may not be of the same chemical element. Triatomic molecules can be linear or nonlinear in shape.
Examples[edit]
Carbon dioxide[edit]
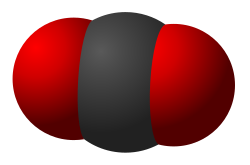
Carbon dioxide (CO_) is a linear triatomic molecule consisting of one carbon atom and two oxygen atoms. It is a significant component of the Earth's atmosphere and is involved in the carbon cycle. Carbon dioxide is a greenhouse gas and plays a crucial role in photosynthesis.
Ozone[edit]

Ozone (O_) is a nonlinear triatomic molecule composed of three oxygen atoms. It is found in the Earth's stratosphere and forms the ozone layer, which absorbs most of the Sun's harmful ultraviolet radiation. Ozone is also present at ground level as a pollutant.
Trihydrogen cation[edit]

The trihydrogen cation (H__) is a positively charged ion consisting of three hydrogen atoms. It is an important species in interstellar space and plays a role in the chemistry of star formation.
Properties[edit]
Triatomic molecules can exhibit a variety of properties depending on their structure and composition. Linear triatomic molecules, such as carbon dioxide, have a straight-line geometry, while nonlinear molecules, like ozone, have a bent shape. These structural differences affect their vibrational and rotational spectra.
Applications[edit]
Triatomic molecules are important in various fields of science and technology. Carbon dioxide is used in carbonated beverages, fire extinguishers, and as a refrigerant. Ozone is used in water purification and air purification systems. The trihydrogen cation is studied in astrophysics and quantum chemistry.
Related pages[edit]
References[edit]
- Atkins, P., & de Paula, J. (2006). Physical Chemistry. Oxford University Press.
- Herzberg, G. (1966). Molecular Spectra and Molecular Structure. Van Nostrand Reinhold.
-
Carbon dioxide molecule
-
Ozone molecule
-
Trihydrogen cation


