Gould–Jacobs reaction: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 24: | Line 24: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:Gould-Jacobs_Reaction.png|Gould–Jacobs reaction | |||
File:Gould-Jacobs_reaction.svg|Gould–Jacobs reaction | |||
File:Gould_Jacobs_mechanism.png|Gould–Jacobs reaction mechanism | |||
File:Example_of_aminoalkylamino_derivatives_of_2,3-dihydrofuroquinolines.png|Example of aminoalkylamino derivatives of 2,3-dihydrofuroquinolines | |||
File:Gould-Jacobs_reaction_on_5-aminoindole.png|Gould–Jacobs reaction on 5-aminoindole | |||
File:Synthesis_of_ethyl_4‐oxo‐8,10‐substituted‐4,8‐dihydropyrimido(1,2‐c)pyrrolo(3,2‐e)pyrimidine‐3‐carboxylates_by_the_Gould-Jacobs_reaction.png|Synthesis of ethyl 4‐oxo‐8,10‐substituted‐4,8‐dihydropyrimido(1,2‐c)pyrrolo(3,2‐e)pyrimidine‐3‐carboxylates by the Gould–Jacobs reaction | |||
</gallery> | |||
Latest revision as of 11:35, 18 February 2025
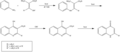
Gould–Jacobs reaction is a chemical reaction used in organic chemistry for the synthesis of quinolines. It was first reported by Maurice Gould and Lewis Jacobs in 1939. The reaction involves the formation of quinolines through the cyclization of an aniline derivative with a β-dicarbonyl compound, typically in the presence of an acid catalyst.
Reaction Mechanism[edit]
The Gould–Jacobs reaction begins with the formation of an amide from the reaction of an aniline derivative with a β-dicarbonyl compound. This step is usually facilitated by an acid catalyst. Subsequent cyclization of the amide intermediate leads to the formation of a dihydroquinoline. Finally, oxidation of the dihydroquinoline yields the quinoline product. The choice of oxidizing agent can vary, but common choices include nitrobenzene and various quinones.
Applications[edit]
Quinolines synthesized through the Gould–Jacobs reaction find applications in various fields, including pharmaceuticals, agrochemicals, and materials science. Quinoline derivatives are known for their antimalarial, antibacterial, and antifungal properties. Additionally, they serve as key intermediates in the synthesis of dyes, organic LEDs, and other functional materials.
Variations[edit]
Several variations of the Gould–Jacobs reaction have been developed to improve its efficiency, yield, and applicability to a broader range of substrates. These include modifications in the choice of acid catalysts, the use of different oxidizing agents, and the employment of alternative reaction conditions (e.g., microwave irradiation). These modifications aim to enhance the reaction's selectivity and to reduce the environmental impact of the synthesis process.
See Also[edit]
References[edit]
<references/>
-
Gould–Jacobs reaction
-
Gould–Jacobs reaction
-
Gould–Jacobs reaction mechanism
-
Example of aminoalkylamino derivatives of 2,3-dihydrofuroquinolines
-
Gould–Jacobs reaction on 5-aminoindole
-
Synthesis of ethyl 4‐oxo‐8,10‐substituted‐4,8‐dihydropyrimido(1,2‐c)pyrrolo(3,2‐e)pyrimidine‐3‐carboxylates by the Gould–Jacobs reaction