Thiomescaline: Difference between revisions
CSV import |
CSV import |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|A synthetic psychedelic compound}} | |||
{{Psychedelic drug}} | |||
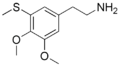
'''Thiomescaline''' is a synthetic psychedelic compound that belongs to the class of [[phenethylamines]]. It is structurally related to [[mescaline]], a naturally occurring psychedelic found in certain cacti, but with a sulfur atom replacing one of the oxygen atoms in the mescaline molecule. This modification results in distinct pharmacological properties. | |||
Thiomescaline | ==Chemical Structure and Properties== | ||
Thiomescaline is chemically known as 2-(3,4,5-trimethoxyphenyl)ethanethioamine. The presence of the sulfur atom in its structure differentiates it from mescaline, which is 3,4,5-trimethoxyphenethylamine. This substitution is responsible for its unique effects and potency. | |||
== | ==Pharmacology== | ||
Thiomescaline acts primarily as a [[serotonin receptor]] agonist, particularly at the 5-HT<sub>2A</sub> receptor, which is believed to be responsible for its psychedelic effects. The compound may also interact with other serotonin receptors, contributing to its overall psychoactive profile. | |||
===Effects=== | |||
The effects of thiomescaline are similar to those of other psychedelics, including altered perception of time and space, visual hallucinations, and changes in mood and thought processes. Users may experience enhanced sensory perception and a sense of interconnectedness with their surroundings. | |||
== | ===Duration=== | ||
The onset of effects typically occurs within 30 to 60 minutes after ingestion, with the peak effects lasting for 4 to 6 hours. The total duration of the experience can last up to 8 hours, depending on the dose and individual metabolism. | |||
The | ==Synthesis== | ||
The synthesis of thiomescaline involves the introduction of a sulfur atom into the mescaline structure. This can be achieved through various chemical reactions, including the use of thioethers or thiols as starting materials. The synthesis requires expertise in organic chemistry and access to specialized laboratory equipment. | |||
== Legal Status == | ==Legal Status== | ||
The legal status of thiomescaline varies by country. In many jurisdictions, it is classified as a controlled substance due to its structural similarity to mescaline and its psychoactive effects. Researchers interested in studying thiomescaline must obtain appropriate licenses and adhere to regulatory guidelines. | |||
==Potential Uses== | |||
While thiomescaline is primarily known for its recreational use, there is interest in its potential therapeutic applications. Like other psychedelics, it may have potential in the treatment of certain mental health conditions, such as [[depression]] and [[post-traumatic stress disorder]] (PTSD). However, more research is needed to fully understand its efficacy and safety in clinical settings. | |||
== | ==Safety and Risks== | ||
As with other psychedelics, the use of thiomescaline carries certain risks. These include the potential for psychological distress, such as anxiety or paranoia, particularly in individuals with a predisposition to mental health disorders. It is important for users to be in a safe and supportive environment when using thiomescaline. | |||
==Related pages== | |||
* [[Mescaline]] | * [[Mescaline]] | ||
* [[Psychedelic drug]] | * [[Psychedelic drug]] | ||
* [[ | * [[Serotonin receptor]] | ||
* [[ | * [[Phenethylamine]] | ||
[[Category:Psychedelic | [[Category:Psychedelic phenethylamines]] | ||
<gallery caption="Thiomescaline"> | |||
File:3-TM.png|3-TM | |||
File:4-TM.png|4-TM | |||
</gallery> | |||
Latest revision as of 05:37, 3 March 2025
A synthetic psychedelic compound
Thiomescaline is a synthetic psychedelic compound that belongs to the class of phenethylamines. It is structurally related to mescaline, a naturally occurring psychedelic found in certain cacti, but with a sulfur atom replacing one of the oxygen atoms in the mescaline molecule. This modification results in distinct pharmacological properties.
Chemical Structure and Properties[edit]
Thiomescaline is chemically known as 2-(3,4,5-trimethoxyphenyl)ethanethioamine. The presence of the sulfur atom in its structure differentiates it from mescaline, which is 3,4,5-trimethoxyphenethylamine. This substitution is responsible for its unique effects and potency.
Pharmacology[edit]
Thiomescaline acts primarily as a serotonin receptor agonist, particularly at the 5-HT2A receptor, which is believed to be responsible for its psychedelic effects. The compound may also interact with other serotonin receptors, contributing to its overall psychoactive profile.
Effects[edit]
The effects of thiomescaline are similar to those of other psychedelics, including altered perception of time and space, visual hallucinations, and changes in mood and thought processes. Users may experience enhanced sensory perception and a sense of interconnectedness with their surroundings.
Duration[edit]
The onset of effects typically occurs within 30 to 60 minutes after ingestion, with the peak effects lasting for 4 to 6 hours. The total duration of the experience can last up to 8 hours, depending on the dose and individual metabolism.
Synthesis[edit]
The synthesis of thiomescaline involves the introduction of a sulfur atom into the mescaline structure. This can be achieved through various chemical reactions, including the use of thioethers or thiols as starting materials. The synthesis requires expertise in organic chemistry and access to specialized laboratory equipment.
Legal Status[edit]
The legal status of thiomescaline varies by country. In many jurisdictions, it is classified as a controlled substance due to its structural similarity to mescaline and its psychoactive effects. Researchers interested in studying thiomescaline must obtain appropriate licenses and adhere to regulatory guidelines.
Potential Uses[edit]
While thiomescaline is primarily known for its recreational use, there is interest in its potential therapeutic applications. Like other psychedelics, it may have potential in the treatment of certain mental health conditions, such as depression and post-traumatic stress disorder (PTSD). However, more research is needed to fully understand its efficacy and safety in clinical settings.
Safety and Risks[edit]
As with other psychedelics, the use of thiomescaline carries certain risks. These include the potential for psychological distress, such as anxiety or paranoia, particularly in individuals with a predisposition to mental health disorders. It is important for users to be in a safe and supportive environment when using thiomescaline.
Related pages[edit]
- Thiomescaline
-
3-TM
-
4-TM