Calcium sulfate: Difference between revisions
CSV import |
CSV import |
||
| Line 45: | Line 45: | ||
{{chemistry-stub}} | {{chemistry-stub}} | ||
==Calcium_sulfate== | |||
<gallery> | |||
File:CaSO4simple.svg|CaSO4 simple structure | |||
File:Calcium_sulfate_hemihydrate.jpg|Calcium sulfate hemihydrate | |||
File:CaSO4.tif|CaSO4 | |||
File:Drierite.jpg|Drierite | |||
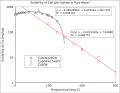
File:Temperature_dependence_calcium_sulfate_solubility.svg|Temperature dependence calcium sulfate solubility | |||
</gallery> | |||
Latest revision as of 21:00, 23 February 2025

Calcium sulfate is a chemical compound with the formula CaSO₄. It is a common laboratory and industrial chemical. In the form of anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry.
Chemical Properties[edit]
Calcium sulfate is a white crystalline solid at room temperature. It is poorly soluble in water. The compound exists in three levels of hydration corresponding to different forms:
- Anhydrous form: anhydrite
- Dihydrate form: gypsum
- Hemihydrate form: plaster of Paris
Production[edit]
Calcium sulfate can be produced by the reaction of calcium carbonate and sulfuric acid. It is also a byproduct of the desulfurization of flue gases in power stations.
Uses[edit]
Calcium sulfate has a variety of uses in different industries:
- In the construction industry, it is used to make plaster, plasterboard, and cement.
- In the food industry, it is used as a coagulant in products like tofu.
- In the medical field, it is used in bone grafting and as a dental impression material.
- It is also used as a desiccant in laboratory and industrial settings.
Health and Safety[edit]
Calcium sulfate is generally considered non-toxic. However, inhalation of dust can cause respiratory irritation. Proper safety measures should be taken when handling the compound in its powdered form.
Related Compounds[edit]
See Also[edit]
References[edit]
<references group="" responsive="1"></references>
External Links[edit]
Calcium_sulfate[edit]
-
CaSO4 simple structure
-
Calcium sulfate hemihydrate
-
CaSO4
-
Drierite
-
Temperature dependence calcium sulfate solubility