Active site: Difference between revisions
CSV import |
CSV import |
||
| Line 66: | Line 66: | ||
[[Category:Enzymes]] | [[Category:Enzymes]] | ||
<gallery> | |||
File:Enzyme_structure.svg|Active_site | |||
File:Lock_and_key.png|Active_site | |||
File:Inducedfit080.png|Active_site | |||
File:NaF.gif|Active_site | |||
File:Hydrogen-bonding-in-water-2D.svg|Active_site | |||
File:Acetone_dipole-dipole.svg|Active_site | |||
File:Cartoon_of_protein_hydrophobic_interaction.jpg|Active_site | |||
File:Catalytic_triad_of_TEV_protease.png|Active_site | |||
File:GSR_Catalytic_Cycle.PNG|Active_site | |||
File:Mechanism_of_peptide_bond_cleavage_in_a-chymotrypsin.svg|Active_site | |||
File:Redox_states_of_FAD.png|Active_site | |||
File:Indinavir,_an_HIV_protease_inhibitor.jpg|Active_site | |||
</gallery> | |||
Latest revision as of 11:55, 18 February 2025
Active Site[edit]
The active site of an enzyme is the region where substrate molecules bind and undergo a chemical reaction. The active site is typically a small pocket or groove on the surface of the enzyme, formed by the three-dimensional arrangement of amino acids. This site is crucial for the enzyme's catalytic activity and specificity.
Structure and Function[edit]
The active site is composed of residues that create a specific environment for the substrate. These residues can participate directly in the chemical transformation of the substrate or help position the substrate correctly. The active site is often highly specific to the substrate, a concept known as the lock and key model.

Induced Fit Model[edit]
The induced fit model suggests that the active site is flexible and can change shape to better fit the substrate upon binding. This model accounts for the dynamic nature of enzyme-substrate interactions.

Catalytic Mechanisms[edit]
Enzymes can employ various mechanisms to catalyze reactions, including:
- Acid-base catalysis: Involves the transfer of protons.
- Covalent catalysis: Involves the formation of a transient covalent bond between the enzyme and the substrate.
- Metal ion catalysis: Utilizes metal ions to stabilize charged intermediates.
- Proximity and orientation effects: Increases the effective concentration of substrates and aligns them for reaction.
Factors Affecting Active Site Function[edit]
Several factors can influence the activity of the active site, including:
- pH and temperature: These can affect the ionization state of the active site residues and the overall enzyme structure.
- Inhibitors: Molecules that can bind to the active site or elsewhere on the enzyme to reduce its activity.
- Cofactors and coenzymes: Non-protein molecules that assist in the catalytic activity of the enzyme.
Examples of Active Sites[edit]
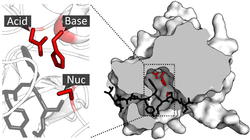
Catalytic Triad[edit]
A common feature in many proteases is the catalytic triad, which consists of three amino acids that work together to perform catalysis. An example is the triad found in TEV protease.
Redox Reactions[edit]
Enzymes like glutathione reductase use active sites to facilitate redox reactions, involving the transfer of electrons.

Related Pages[edit]
Gallery[edit]
-
Structure of an enzyme showing the active site.
-
Illustration of enzyme inhibition.
-
Hydrogen bonding in water, relevant to enzyme interactions.
-
Dipole-dipole interactions in enzyme-substrate binding.
-
Hydrophobic interactions in protein structure.
-
Mechanism of peptide bond cleavage in _-chymotrypsin.
-
Redox states of FAD, a cofactor in enzymatic reactions.
-
Active_site
-
Active_site
-
Active_site
-
Active_site
-
Active_site
-
Active_site
-
Active_site
-
Active_site
-
Active_site
-
Active_site
-
Active_site
-
Active_site











