Naphthoquinone: Difference between revisions
CSV import |
CSV import |
||
| Line 24: | Line 24: | ||
{{stub}} | {{stub}} | ||
{{dictionary-stub1}} | {{dictionary-stub1}} | ||
== Naphthoquinone == | |||
<gallery> | |||
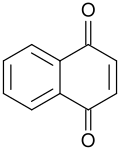
File:1,4-Naphthoquinone.svg|1,4-Naphthoquinone structure | |||
File:Juglone.png|Juglone structure | |||
</gallery> | |||
Latest revision as of 01:49, 17 February 2025
Naphthoquinone is a class of organic compounds that are derived from naphthalene. They are part of the larger quinone family and are often used in dyes, pigments, and as biological electron acceptors.
Chemistry[edit]
Naphthoquinones are a type of quinone that are derived from naphthalene. They are characterized by the presence of two carbonyl groups (C=O) on a naphthalene ring. There are two isomers of naphthoquinone, 1,2-naphthoquinone and 1,4-naphthoquinone, which differ in the position of the carbonyl groups on the naphthalene ring.
Uses[edit]
Naphthoquinones are used in a variety of applications. They are used as dyes and pigments due to their ability to form brightly colored compounds. They are also used as biological electron acceptors in certain types of bacteria and fungi.
Biological Role[edit]
In addition to their industrial uses, naphthoquinones also play a role in biology. They are involved in the electron transport chain in bacteria and fungi, where they act as electron acceptors. Some naphthoquinones are also used in the synthesis of vitamins K and E.
Health Effects[edit]
Exposure to naphthoquinones can have a variety of health effects. They can cause skin and eye irritation, and prolonged exposure can lead to more serious health problems such as liver damage and cancer.