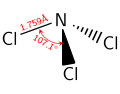Nitrogen trichloride: Difference between revisions
CSV import |
No edit summary |
||
| Line 27: | Line 27: | ||
{{Chem-stub}} | {{Chem-stub}} | ||
== Nitrogen_trichloride == | == Nitrogen_trichloride == | ||
<gallery> | <gallery> | ||
Latest revision as of 00:47, 17 March 2025
Nitrogen Trichloride is a chemical compound consisting of nitrogen and chlorine. It is a yellow, oily liquid that is most commonly used in the bleaching of flour and in the purification of water.
Chemical Properties[edit]
Nitrogen trichloride is a highly reactive, unstable compound. It is a strong oxidizing agent and can explode on contact with organic materials. It is soluble in water and decomposes in sunlight, releasing nitrogen and chlorine gases.
Production[edit]
Nitrogen trichloride is produced by the reaction of ammonia with chlorine. This reaction is exothermic and can be dangerous if not controlled properly. The reaction produces a mixture of nitrogen trichloride, nitrogen monochloride, and nitrogen dichloride.
Uses[edit]
The primary use of nitrogen trichloride is in the bleaching of flour. It is also used in the purification of water, as it is effective at killing bacteria and other microorganisms. However, due to its instability and reactivity, it is not commonly used in industrial applications.
Health Effects[edit]
Exposure to nitrogen trichloride can cause irritation to the eyes, skin, and respiratory system. It can also cause nausea, headache, and dizziness. Long-term exposure can lead to chronic respiratory problems.
Safety[edit]
Due to its reactivity and instability, nitrogen trichloride must be handled with care. It should be stored in a cool, dry place away from heat and open flames. It should also be kept away from organic materials, as it can react violently with them.
See Also[edit]
Nitrogen_trichloride[edit]
-
NCl3 dimensions
-
Nitrogen trichloride 3D vdW
-
Nitrogen trichloride