Tautomer: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 30: | Line 30: | ||
{{Chem-stub}} | {{Chem-stub}} | ||
<gallery> | |||
File:Amino acid zwitterions.svg|Amino acid zwitterions | |||
File:Tautomers.svg|Tautomers | |||
File:Acetylacetone keto-enol tautomerism.svg|Acetylacetone keto-enol tautomerism | |||
File:Guanidinium-ion-canonical-forms-2D-skeletal..png|Guanidinium ion canonical forms | |||
File:Glucose Fisher to Haworth.gif|Glucose Fisher to Haworth | |||
File:Oxepin-benzene oxide.png|Oxepin benzene oxide | |||
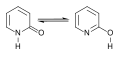
File:2-pyridone-chemical-tautomer.svg|2-pyridone chemical tautomer | |||
</gallery> | |||
Revision as of 06:18, 3 March 2025
Tautomerism is a phenomenon in chemistry where molecules with the same molecular formula exist in multiple forms by the shifting of atomic positions within the molecule. These different forms, known as tautomers, are structural isomers that rapidly interconvert, typically in a dynamic equilibrium. The most common type of tautomerism involves the relocation of a proton (H+) and is called prototropic tautomerism. Other types include valence tautomerism and ring-chain tautomerism, among others. Tautomerism plays a crucial role in various biological processes and has significant implications in organic synthesis, drug design, and biochemistry.
Types of Tautomerism
Tautomerism can be classified into several types based on the nature of the atomic or group transfer. The most notable types include:
- Prototropic Tautomerism: This is the most common form, where tautomers differ by the position of a proton and a double bond. The keto-enol tautomerism, where a ketone form (keto) and an enolic form (enol) interconvert, is a classic example.
- Valence Tautomerism: In this type, the rearrangement involves changes in the connectivity of electrons within a molecule, leading to different bonding patterns.
- Ring-Chain Tautomerism: This involves the interconversion between cyclic and acyclic forms. It is common in sugars and other cyclic compounds.
- Tautomeric Pairs: Specific pairs of tautomers, such as imine-enamine, lactam-lactim, and nitroso-oxime, demonstrate the diversity of tautomerism.
Mechanism and Conditions
The tautomerization process involves the breaking and forming of chemical bonds, typically under the influence of a catalyst or specific conditions like pH changes. The mechanism can vary significantly depending on the type of tautomerism and the involved functional groups. For prototropic tautomerism, the presence of acidic or basic conditions can facilitate the proton transfer.
Biological Significance
Tautomerism has profound implications in biology. The tautomeric forms of nucleobases in DNA can lead to mutations during DNA replication. Additionally, enzyme specificity and drug-receptor interactions can be influenced by the tautomeric states of molecules, affecting the efficacy and metabolism of pharmaceuticals.
Applications in Organic Synthesis and Drug Design
In organic chemistry, controlling tautomerism is essential for the synthesis of desired compounds. Tautomeric equilibrium can influence the reactivity and stability of intermediates. In drug design, understanding tautomerism is crucial for predicting the bioactive form of a molecule, optimizing drug-target interactions, and minimizing off-target effects.
See Also
-
Amino acid zwitterions
-
Tautomers
-
Acetylacetone keto-enol tautomerism
-
Guanidinium ion canonical forms
-
Glucose Fisher to Haworth
-
Oxepin benzene oxide
-
2-pyridone chemical tautomer