Hemiaminal: Difference between revisions
CSV import |
CSV import |
||
| Line 33: | Line 33: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
== Hemiaminal gallery == | |||
<gallery> | |||
File:Hemiaminal-2D-skeletal.png|Hemiaminal 2D skeletal | |||
File:Aminomethanol.jpg|Aminomethanol | |||
File:CarbazoleFormaldehydeReaction.png|Carbazole Formaldehyde Reaction | |||
File:Hemiaminalformationinsaxitoxinsynthesis.png|Hemiaminal formation in saxitoxin synthesis | |||
</gallery> | |||
Latest revision as of 06:08, 3 March 2025
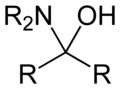
Hemiaminal refers to a functional group or a chemical compound that contains both an alcohol and an amine group attached to the same carbon atom. The general formula for a hemiaminal is R2C(OH)NR2, where R can be a hydrogen atom or an organic substituent. Hemiaminals are considered intermediates in the reaction pathway between aldehydes or ketones and amines, leading to the formation of imines or aminals, depending on the reaction conditions and the reactants involved.
Structure and Formation[edit]
Hemiaminals are characterized by the presence of a hydroxyl (-OH) group and an amine (-NR2) group attached to the same carbon atom. This structure is formed through the nucleophilic addition of an amine to an aldehyde or ketone. The process typically involves the initial formation of a carbinolamine, which can then undergo further reactions to form a hemiaminal.
The formation of a hemiaminal can be represented by the following general reaction:
R2C=O + HNR2 → R2C(OH)NR2
where R2C=O represents an aldehyde or ketone, and HNR2 represents an amine.
Reactivity and Significance[edit]
Hemiaminals are generally unstable and can undergo several possible reactions. They can dehydrate to form imines, a process that is often catalyzed by acids or bases. Alternatively, hemiaminals can react with another molecule of amine to form aminals, especially in the presence of excess amine.
The instability of hemiaminals makes them challenging to isolate and study. However, they are important intermediates in organic synthesis and biochemistry. For example, the formation of hemiaminals is a key step in the biosynthesis of many alkaloids and other nitrogen-containing natural products.
Applications[edit]
While hemiaminals themselves are not typically isolated due to their instability, the understanding of their formation and reactivity is crucial in organic synthesis. They play a role in the synthesis of imines, which are valuable intermediates in the production of amines, polymers, and pharmaceuticals. Additionally, the study of hemiaminal formation and stability is important in the development of new synthetic methodologies and the understanding of biochemical processes.
Related Compounds[edit]
- Carbinolamine: A related intermediate that contains a hydroxyl group attached to a carbon atom, which is also bonded to an amine group. Carbinolamines can dehydrate to form hemiaminals.
- Imines: Compounds formed from the dehydration of hemiaminals, containing a carbon-nitrogen double bond.
- Aminals: Compounds formed from the reaction of hemiaminals with an additional amine molecule, characterized by two nitrogen atoms bonded to the same carbon atom.
See Also[edit]
Hemiaminal gallery[edit]
-
Hemiaminal 2D skeletal
-
Aminomethanol
-
Carbazole Formaldehyde Reaction
-
Hemiaminal formation in saxitoxin synthesis