Cubic crystal system: Difference between revisions
CSV import |
CSV import |
||
| Line 45: | Line 45: | ||
[[Category:Materials Science]] | [[Category:Materials Science]] | ||
[[Category:Solid-State Physics]] | [[Category:Solid-State Physics]] | ||
== Cubic crystal system gallery == | |||
<gallery> | |||
File:Pyrite Cubes.JPG|Pyrite Cubes | |||
File:Kubisches Kristallsystem.jpg|Kubisches Kristallsystem | |||
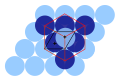
File:FCC primative-cubic cells.svg|FCC primitive cubic cells | |||
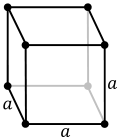
File:Cubic.svg|Cubic | |||
File:Cubic-body-centered.svg|Cubic body-centered | |||
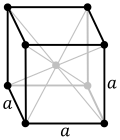
File:Cubic-face-centered.svg|Cubic face-centered | |||
File:visualisation diamond cubic.svg|Visualisation diamond cubic | |||
File:CsCl crystal.svg|CsCl crystal | |||
File:Cesium Chloride.jpg|Cesium Chloride | |||
File:NaCl octahedra in crystal.svg|NaCl octahedra in crystal | |||
</gallery> | |||
Latest revision as of 05:45, 3 March 2025
Cubic Crystal System[edit]
The cubic crystal system is one of the seven crystal systems in mineralogy and crystallography. It is characterized by a three-dimensional arrangement of atoms or molecules that form a cube-like structure. The cubic crystal system is known for its symmetry and regularity, making it an important topic in the study of crystals.
Characteristics[edit]
In the cubic crystal system, the lattice structure is composed of equally spaced points that form a three-dimensional grid. The lattice points represent the positions of atoms or molecules within the crystal. The cubic system has three axes of equal length, intersecting at right angles, and all angles are 90 degrees.
Types of Cubic Crystals[edit]
There are three main types of cubic crystals: simple cubic, body-centered cubic (bcc), and face-centered cubic (fcc).
- Simple Cubic: In a simple cubic crystal, each lattice point is occupied by a single atom or molecule. The atoms are arranged in a simple cubic lattice, with one atom at each corner of the cube.
- Body-Centered Cubic (bcc): In a body-centered cubic crystal, there is an additional atom at the center of the cube, in addition to the atoms at the corners. This creates a more complex lattice structure.
- Face-Centered Cubic (fcc): In a face-centered cubic crystal, there is an additional atom at the center of each face of the cube, in addition to the atoms at the corners. This results in a highly symmetrical lattice structure.
Examples[edit]
Many common materials exhibit cubic crystal structures. Some examples include:
- Sodium Chloride (NaCl): Commonly known as table salt, sodium chloride crystallizes in a face-centered cubic structure.
- Iron (Fe): Iron crystallizes in a body-centered cubic structure, which contributes to its unique properties such as strength and magnetism.
- Diamond: Although diamond is composed of carbon atoms, it has a cubic crystal structure due to the arrangement of its atoms.
Importance in Science and Technology[edit]
The cubic crystal system has significant implications in various scientific and technological fields. Its regular and symmetrical structure allows for the prediction and understanding of material properties, such as electrical conductivity, thermal conductivity, and optical behavior. This knowledge is crucial in fields such as materials science, solid-state physics, and engineering.
See Also[edit]
- Crystallography: The study of crystals and their structures. - Crystal Systems: An overview of the seven crystal systems. - Mineralogy: The study of minerals and their properties.
References[edit]
<references />
Cubic crystal system gallery[edit]
-
Pyrite Cubes
-
Kubisches Kristallsystem
-
FCC primitive cubic cells
-
Cubic
-
Cubic body-centered
-
Cubic face-centered
-
Visualisation diamond cubic
-
CsCl crystal
-
Cesium Chloride
-
NaCl octahedra in crystal