Sandmeyer reaction: Difference between revisions
CSV import |
CSV import |
||
| Line 28: | Line 28: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
== Sandmeyer reaction gallery == | |||
<gallery> | |||
File:Sandmeyer.jpg|Sandmeyer | |||
File:Sandmeyerbromination.png|Sandmeyerbromination | |||
File:Sandmeyer Reactions.svg|Sandmeyer Reactions | |||
File:Bromination using Sandmeyer.png|Bromination using Sandmeyer | |||
File:Bf4sm.png|Bf4sm | |||
File:CyanationSandmeyer.png|Cyanation Sandmeyer | |||
File:Cyanation using the Sandmeyer reaction.png|Cyanation using the Sandmeyer reaction | |||
File:Trifluoromethylation using Sandmeyer.png|Trifluoromethylation using Sandmeyer | |||
File:Hydroxylation using Sandmeyer reaction.png|Hydroxylation using Sandmeyer reaction | |||
</gallery> | |||
Latest revision as of 05:08, 3 March 2025
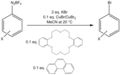
Sandmeyer Reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts. This reaction is an important tool in the field of organic chemistry, particularly in the synthesis of complex molecules. The Sandmeyer Reaction involves the replacement of the diazonium group (-N_2^+) of an aryl diazonium salt with a nucleophile, often a halide. This process is facilitated by the presence of copper(I) salts (e.g., CuCl, CuBr) as catalysts. The reaction is named after the Swiss chemist Traugott Sandmeyer, who first reported it in 1884.
Mechanism[edit]
The mechanism of the Sandmeyer Reaction involves several key steps. Initially, the aryl diazonium salt reacts with the copper(I) catalyst to form a reactive aryl copper intermediate. This intermediate then undergoes nucleophilic substitution with the halide ion present in the reaction mixture, leading to the formation of the aryl halide product. The exact mechanism can vary depending on the specific conditions and substrates used in the reaction.
Applications[edit]
The Sandmeyer Reaction is widely used in organic synthesis for the preparation of aryl halides, which are valuable intermediates in the synthesis of a wide range of organic compounds. This reaction is particularly useful for introducing halogen atoms into aromatic rings, which can then undergo further transformations, such as cross-coupling reactions. The ability to efficiently synthesize aryl halides using the Sandmeyer Reaction has made it a staple technique in pharmaceutical and agrochemical research and development.
Variations[edit]
Several variations of the Sandmeyer Reaction exist, which allow for the introduction of different functional groups into the aromatic ring. For example, the Gattermann Reaction, which is closely related to the Sandmeyer Reaction, is used to introduce aldehyde groups into aromatic rings. Other variations include the introduction of cyano, nitro, and other groups, expanding the versatility of the Sandmeyer Reaction in organic synthesis.
Limitations[edit]
While the Sandmeyer Reaction is a powerful tool for the synthesis of aryl halides, it does have some limitations. The reaction conditions can sometimes lead to the decomposition of the diazonium salt, reducing the yield of the desired product. Additionally, the use of copper(I) salts as catalysts can introduce metallic impurities into the final product, which may be undesirable in certain applications.
See Also[edit]
- Aryl halide
- Diazonium compound
- Nucleophilic substitution
- Gattermann Reaction
- Cross-coupling reaction
References[edit]
<references/>
Sandmeyer reaction gallery[edit]
-
Sandmeyer
-
Sandmeyerbromination
-
Sandmeyer Reactions
-
Bromination using Sandmeyer
-
Bf4sm
-
Cyanation Sandmeyer
-
Cyanation using the Sandmeyer reaction
-
Trifluoromethylation using Sandmeyer
-
Hydroxylation using Sandmeyer reaction