Alfred Werner: Difference between revisions
CSV import |
CSV import |
||
| Line 4: | Line 4: | ||
File:Trans-dichlorotetraamminecobalt(III).png|Trans-dichlorotetraamminecobalt(III) | File:Trans-dichlorotetraamminecobalt(III).png|Trans-dichlorotetraamminecobalt(III) | ||
</gallery> | </gallery> | ||
== Alfred Werner == | |||
'''Alfred Werner''' (12 December 1866 – 15 November 1919) was a Swiss chemist who was awarded the Nobel Prize in Chemistry in 1913 for his work on the [[coordination chemistry|coordination theory]] of [[transition metal]] complexes. Werner's pioneering research laid the foundation for modern coordination chemistry and significantly advanced the understanding of the structure of [[inorganic compounds]]. | |||
== Early Life and Education == | |||
Alfred Werner was born in [[Mulhouse]], which was then part of the [[Alsace]] region in [[France]]. He was the youngest of four children in a family of modest means. Werner showed an early interest in chemistry and pursued his studies at the [[Swiss Federal Institute of Technology in Zurich]] (ETH Zurich), where he completed his doctorate in 1890 under the supervision of [[Arthur Rudolf Hantzsch]]. | |||
== Academic Career == | |||
After completing his doctorate, Werner remained at ETH Zurich as a lecturer and later became a professor of chemistry. His research focused on the structure of [[coordination compounds]], which are complexes formed between metal ions and surrounding molecules or ions, known as [[ligands]]. | |||
== Coordination Theory == | |||
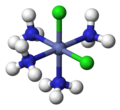
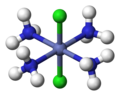
Werner's most significant contribution to chemistry was his coordination theory, which he proposed in 1893. This theory explained the [[valency]] and spatial arrangement of atoms in coordination compounds. Werner proposed that metal ions could form primary and secondary valency bonds with ligands, leading to the formation of complex ions with specific geometric arrangements. | |||
Werner's theory challenged the prevailing ideas of the time, which were based on [[valence bond theory]] and the [[octet rule]]. He introduced the concept of coordination number, which is the number of ligand atoms directly bonded to the central metal ion. Werner's work demonstrated that coordination compounds could exhibit various geometries, such as [[octahedral]], [[tetrahedral]], and [[square planar]] arrangements. | |||
== Nobel Prize and Legacy == | |||
In 1913, Alfred Werner was awarded the [[Nobel Prize in Chemistry]] for his coordination theory, making him the first inorganic chemist to receive this honor. His work provided a new understanding of the structure and bonding of inorganic compounds, influencing subsequent research in the field. | |||
Werner's coordination theory laid the groundwork for the development of [[crystal field theory]] and [[ligand field theory]], which further advanced the study of transition metal complexes. His contributions to chemistry are commemorated by the naming of the [[Werner complex]] and the [[Werner coordination number]]. | |||
== Personal Life == | |||
Alfred Werner married Emma Giesker in 1894, and they had one son. Despite his scientific achievements, Werner's health declined in his later years, and he passed away in Zurich in 1919 at the age of 52. | |||
== Related Pages == | |||
* [[Coordination chemistry]] | |||
* [[Nobel Prize in Chemistry]] | |||
* [[Transition metal]] | |||
* [[Inorganic chemistry]] | |||
{{Nobel laureates in Chemistry 1910-1919}} | |||
{{Authority control}} | |||
[[Category:1866 births]] | |||
[[Category:1919 deaths]] | |||
[[Category:Swiss chemists]] | |||
[[Category:Nobel laureates in Chemistry]] | |||
[[Category:ETH Zurich faculty]] | |||
Latest revision as of 00:39, 19 February 2025
-
Alfred Werner
-
Cis-dichlorotetraamminecobalt(III)
-
Trans-dichlorotetraamminecobalt(III)
Alfred Werner[edit]
Alfred Werner (12 December 1866 – 15 November 1919) was a Swiss chemist who was awarded the Nobel Prize in Chemistry in 1913 for his work on the coordination theory of transition metal complexes. Werner's pioneering research laid the foundation for modern coordination chemistry and significantly advanced the understanding of the structure of inorganic compounds.
Early Life and Education[edit]
Alfred Werner was born in Mulhouse, which was then part of the Alsace region in France. He was the youngest of four children in a family of modest means. Werner showed an early interest in chemistry and pursued his studies at the Swiss Federal Institute of Technology in Zurich (ETH Zurich), where he completed his doctorate in 1890 under the supervision of Arthur Rudolf Hantzsch.
Academic Career[edit]
After completing his doctorate, Werner remained at ETH Zurich as a lecturer and later became a professor of chemistry. His research focused on the structure of coordination compounds, which are complexes formed between metal ions and surrounding molecules or ions, known as ligands.
Coordination Theory[edit]
Werner's most significant contribution to chemistry was his coordination theory, which he proposed in 1893. This theory explained the valency and spatial arrangement of atoms in coordination compounds. Werner proposed that metal ions could form primary and secondary valency bonds with ligands, leading to the formation of complex ions with specific geometric arrangements.
Werner's theory challenged the prevailing ideas of the time, which were based on valence bond theory and the octet rule. He introduced the concept of coordination number, which is the number of ligand atoms directly bonded to the central metal ion. Werner's work demonstrated that coordination compounds could exhibit various geometries, such as octahedral, tetrahedral, and square planar arrangements.
Nobel Prize and Legacy[edit]
In 1913, Alfred Werner was awarded the Nobel Prize in Chemistry for his coordination theory, making him the first inorganic chemist to receive this honor. His work provided a new understanding of the structure and bonding of inorganic compounds, influencing subsequent research in the field.
Werner's coordination theory laid the groundwork for the development of crystal field theory and ligand field theory, which further advanced the study of transition metal complexes. His contributions to chemistry are commemorated by the naming of the Werner complex and the Werner coordination number.
Personal Life[edit]
Alfred Werner married Emma Giesker in 1894, and they had one son. Despite his scientific achievements, Werner's health declined in his later years, and he passed away in Zurich in 1919 at the age of 52.