Tacticity: Difference between revisions
CSV import |
CSV import |
||
| Line 24: | Line 24: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:Syndiotactic_polypropene.png|Tacticity | |||
File:Meso_diad.PNG|Tacticity | |||
File:Racemo_diad.PNG|Tacticity | |||
File:Mm_triad.png|Tacticity | |||
File:Rr_triad.PNG|Tacticity | |||
File:Rm_triad.PNG|Tacticity | |||
File:IUPAC_definition_for_a_tactic_block_in_a_polymer.png|Tacticity | |||
File:Isotactic-A-2D-skeletal.png|Tacticity | |||
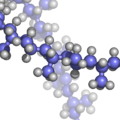
File:Isotactic-polypropylene-3D-balls.png|Tacticity | |||
File:Syndiotactic-2D-skeletal.png|Tacticity | |||
File:Syndiotactic-polypropylene-3D-balls.png|Tacticity | |||
File:Atactic-2D-skeletal.png|Tacticity | |||
</gallery> | |||
Latest revision as of 12:13, 18 February 2025
Tacticity refers to the relative stereochemistry of chiral centers in polymers. It is a key concept in polymer chemistry and materials science, particularly when discussing the physical properties of polymers. Tacticity influences the degree of crystallinity, melting point, and the mechanical properties of polymers, making it a critical factor in the design and application of polymeric materials.
Overview[edit]
Tacticity describes the arrangement of the side chains along the backbone of a polymer molecule. The orientation of these side chains can significantly affect the polymer's physical properties. There are three primary types of tacticity: isotactic, syndiotactic, and atactic.
- Isotactic polymers have all their substituents (side chains) on the same side of the polymer chain. This regularity allows for a higher degree of crystallinity, often leading to materials that are stiffer and have higher melting points.
- Syndiotactic polymers feature substituents alternating sides along the polymer chain. This arrangement also permits a degree of crystallinity, though typically less than isotactic polymers, resulting in materials with somewhat different physical properties.
- Atactic polymers have random substituent arrangements. This randomness usually prevents the polymer chains from packing efficiently into a crystalline structure, resulting in amorphous materials with lower melting points and less rigidity.
Measurement and Analysis[edit]
The tacticity of a polymer can be determined using various spectroscopic techniques, including NMR spectroscopy. NMR allows for the observation of the spatial arrangement of atoms within the polymer, providing detailed information on its tacticity.
Impact on Properties[edit]
The physical properties of polymers, such as their thermal and mechanical behavior, are profoundly influenced by their tacticity. For example, isotactic polymers, with their higher degree of crystallinity, tend to have higher tensile strength and melting points compared to their atactic counterparts. This makes isotactic polymers suitable for applications requiring materials with high strength and thermal resistance.
Applications[edit]
Tacticity plays a crucial role in determining the suitability of polymers for various applications. For instance, isotactic polypropylene is widely used in packaging, fibers, and automotive parts due to its high strength and resistance to deformation under heat. Syndiotactic polystyrene, with its unique blend of properties, finds applications in electronics and construction.
Conclusion[edit]
Understanding the concept of tacticity and its impact on the physical properties of polymers is essential for the design and application of polymeric materials. The ability to control the stereochemistry of polymer chains allows scientists and engineers to tailor materials for specific applications, highlighting the importance of tacticity in materials science and polymer chemistry.
-
Tacticity
-
Tacticity
-
Tacticity
-
Tacticity
-
Tacticity
-
Tacticity
-
Tacticity
-
Tacticity
-
Tacticity
-
Tacticity
-
Tacticity
-
Tacticity