Anthrax toxin: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 29: | Line 29: | ||
{{Toxin-stub}} | {{Toxin-stub}} | ||
{{Bacteria-stub}} | {{Bacteria-stub}} | ||
<gallery> | |||
File:PDB_1zxv_EBI.jpg|Structure of anthrax toxin lethal factor | |||
File:PDB_1pwq_EBI.jpg|Structure of anthrax toxin protective antigen | |||
File:AnthraxBacteria.jpg|Bacillus anthracis bacteria | |||
File:Anthraxtoxins_diagram_en.png|Diagram of anthrax toxins | |||
File:ANTHRA_1.JPG|Anthrax toxin | |||
File:PA-63-octamer_3HVD.png|PA-63 octamer structure | |||
File:Anthrax_toxin_protein_key_motif.svg|Key motif in anthrax toxin protein | |||
File:Translocation_of_anthrax_toxin_protein.jpg|Translocation of anthrax toxin protein | |||
</gallery> | |||
Latest revision as of 11:21, 18 February 2025
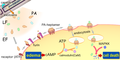
Anthrax toxin is a three-protein exotoxin secreted by virulent strains of the bacterium, Bacillus anthracis, the etiological agent of Anthrax. The toxin was first discovered by Harry Smith in 1954. It is composed of three distinct proteins, namely: Protective antigen, Edema factor, and Lethal factor. Each of these proteins plays a unique role in the pathogenicity of B. anthracis.
Protective Antigen[edit]
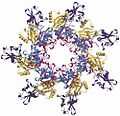
The Protective antigen (PA) is an 83-kDa protein that binds to two host cell receptors, ANTXR1 and ANTXR2. After binding, it is cleaved by a host protease into a 63-kDa protein that oligomerizes into a ring-shaped heptamer or octamer. This structure forms a pre-pore that can bind up to three molecules of EF and/or LF.
Edema Factor[edit]
The Edema factor (EF) is a calmodulin-dependent adenylate cyclase that increases the concentration of cyclic AMP (cAMP) in the host cell. High levels of cAMP interfere with the signaling pathways of the host cell, leading to edema (swelling), immune evasion, and other effects.
Lethal Factor[edit]
The Lethal factor (LF) is a zinc-dependent metalloprotease that cleaves the N-terminus of most mitogen-activated protein kinase kinases (MAPKKs or MEKs), disrupting cellular signaling and leading to cell death.
Mechanism of Action[edit]
The anthrax toxin enters its host cell by receptor-mediated endocytosis. The PA component of the toxin binds to its receptors on the host cell and is cleaved by a protease, forming a pre-pore that can bind EF and/or LF. The host cell then endocytoses the toxin-receptor complex, and the acidic environment of the endosome triggers a conformational change in PA, causing it to insert into the endosomal membrane and form a pore. EF and LF then translocate through this pore into the cytosol, where they exert their toxic effects.
Clinical Significance[edit]
Anthrax toxin plays a key role in the virulence of B. anthracis and is the major cause of damage during Anthrax infections. It is also the target of the anthrax vaccines and many therapeutic drugs.
See Also[edit]
This toxin-related article is a stub. You can help WikiMD by expanding it.
-
Structure of anthrax toxin lethal factor
-
Structure of anthrax toxin protective antigen
-
Bacillus anthracis bacteria
-
Diagram of anthrax toxins
-
Anthrax toxin
-
PA-63 octamer structure
-
Key motif in anthrax toxin protein
-
Translocation of anthrax toxin protein