Amyl alcohol: Difference between revisions
CSV import |
CSV import |
||
| Line 37: | Line 37: | ||
[[Category:Flavoring substances]] | [[Category:Flavoring substances]] | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:Pentan-1-ol-2D-skeletal.png|Pentan-1-ol | |||
File:2-Methyl-1-butanol.svg|2-Methyl-1-butanol | |||
File:Isoamyl_alcohol.svg|Isoamyl alcohol | |||
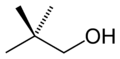
File:Neopentyl-alcohol-2D-skeletal.png|Neopentyl alcohol | |||
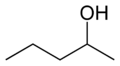
File:Pentan-2-ol-2D-skeletal.png|Pentan-2-ol | |||
File:3-methylbutan-2-ol-2D-skeletal.png|3-Methylbutan-2-ol | |||
File:Pentan-3-ol-2D-skeletal.png|Pentan-3-ol | |||
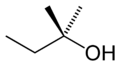
File:Tert-pentyl-alcohol-2D-skeletal.png|Tert-pentyl alcohol | |||
</gallery> | |||
Latest revision as of 11:19, 18 February 2025
Amyl alcohol (also known as pentanol) is a group of organic compounds with the formula C5H11OH. These compounds are primarily derived from natural fats and oils. They are colorless, liquid alcohols that are used in a variety of industrial applications.
Chemical Structure[edit]
Amyl alcohols have the general formula C5H11OH. This means they have five carbon atoms (C), eleven hydrogen atoms (H), and one hydroxyl group (-OH). The hydroxyl group is what classifies these compounds as alcohols.
Types of Amyl Alcohol[edit]
There are eight isomers of amyl alcohol, including:
- 1-Pentanol (n-pentanol, n-amyl alcohol)
- 2-Pentanol (sec-amyl alcohol)
- 3-Pentanol
- 2-Methyl-1-butanol (isopentyl alcohol)
- 3-Methyl-1-butanol (isoamyl alcohol)
- 2-Methyl-2-butanol (tert-amyl alcohol)
- 3-Methyl-2-butanol
- 2,2-Dimethyl-1-propanol
Uses[edit]
Amyl alcohols are used in a variety of industrial applications. They are used as solvents in the production of other chemicals, and as ingredients in perfumes and other fragrances. They are also used in the production of pharmaceuticals, and in the food and beverage industry as flavoring agents.
Health Effects[edit]
Exposure to amyl alcohols can cause a variety of health effects. Inhalation can cause respiratory irritation, dizziness, and nausea. Ingestion can cause gastrointestinal irritation, vomiting, and diarrhea. Long-term exposure can cause damage to the liver and kidneys.
See Also[edit]
References[edit]
<references />
-
Pentan-1-ol
-
2-Methyl-1-butanol
-
Isoamyl alcohol
-
Neopentyl alcohol
-
Pentan-2-ol
-
3-Methylbutan-2-ol
-
Pentan-3-ol
-
Tert-pentyl alcohol