Naphthomycin: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 24: | Line 24: | ||
{{pharmacology-stub}} | {{pharmacology-stub}} | ||
<gallery> | |||
File:Naphthomycin_A.svg|Naphthomycin A | |||
File:Naphthomycin_B.svg|Naphthomycin B | |||
File:Naphthomycin_C.svg|Naphthomycin C | |||
File:Naphthomycin_D.svg|Naphthomycin D | |||
File:Naphthomycin_E.svg|Naphthomycin E | |||
File:Naphthomycin_F.svg|Naphthomycin F | |||
File:Naphthomycin_G.svg|Naphthomycin G | |||
</gallery> | |||
Latest revision as of 11:11, 18 February 2025
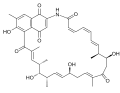
Naphthomycin is a type of antibiotic that belongs to the macrolide family. It is a complex polyketide natural product that is produced by the bacterium Streptomyces. Naphthomycin has been found to have potent antibacterial activity against a wide range of Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE).
Structure and Synthesis[edit]
Naphthomycin is a 26-membered macrolide with a unique naphthalene moiety. The naphthalene ring is thought to be synthesized via a type II polyketide synthase (PKS) pathway, while the macrolide ring is synthesized via a type I PKS pathway. The final assembly of the two parts is believed to be catalyzed by a glycosyltransferase enzyme.
Mechanism of Action[edit]
Like other macrolide antibiotics, naphthomycin works by binding to the 50S ribosomal subunit of bacteria, thereby inhibiting protein synthesis. However, the presence of the naphthalene ring in naphthomycin gives it additional antibacterial properties, such as the ability to disrupt bacterial cell membranes.
Clinical Use[edit]
Naphthomycin has been shown to be effective against a variety of Gram-positive bacteria in vitro. However, its clinical use has been limited due to its complex structure and the difficulty of synthesizing it in large quantities. Research is ongoing to develop more efficient methods of synthesizing naphthomycin and to explore its potential use in treating drug-resistant bacterial infections.
See Also[edit]
References[edit]
<references />
-
Naphthomycin A
-
Naphthomycin B
-
Naphthomycin C
-
Naphthomycin D
-
Naphthomycin E
-
Naphthomycin F
-
Naphthomycin G