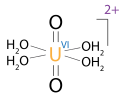Ion: Difference between revisions
CSV import Tag: Manual revert |
CSV import |
||
| Line 29: | Line 29: | ||
{{stub}} | {{stub}} | ||
<gallery> | |||
File:Ionic_bonding.svg|Ionic bonding illustration | |||
File:Ions.svg|Diagram of ions | |||
File:Ion_chamber_operation.gif|Operation of an ion chamber | |||
File:Electron_avalanche.gif|Electron avalanche process | |||
File:Ions_notation.svg|Ions notation | |||
File:Ions_notation2.svg|Alternative ions notation | |||
File:Nitrate-ion-elpot.png|Nitrate ion electrostatic potential | |||
</gallery> | |||
Latest revision as of 11:06, 18 February 2025
Ion
An ion is an atom or molecule that has a net electrical charge. Since the charge of the electron (considered negative by convention) is equal and opposite to that of the proton (considered positive by convention), the net charge of an ion is non-zero due to its total number of electrons being unequal to its total number of protons.
Types of ions[edit]
Ions can be created by both chemical and physical means. In chemical terms, if a neutral atom loses one or more electrons, it has a net positive charge and is known as a cation. If an atom gains electrons, it has a net negative charge and is known as an anion.
Formation of ions[edit]
Ions are formed by the addition or subtraction of electrons from atoms, leading to the formation of cations and anions. This process is known as ionization.
Uses of ions[edit]
Ions have many uses in our daily life. They are used in everything from the functioning of the nervous system, to the treatment of cancer, to the operation of computers.