Corey–Fuchs reaction: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import Tags: mobile edit mobile web edit |
||
| Line 29: | Line 29: | ||
[[Category:Name reactions]] | [[Category:Name reactions]] | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:Corey-Fuchs_Reaction_Scheme.png|Corey–Fuchs reaction scheme | |||
File:Corey-Fuchs_reaction_step_1.svg|Corey–Fuchs reaction step 1 | |||
File:Corey-Fuchs_reaction_step_2.svg|Corey–Fuchs reaction step 2 | |||
File:Corey-Fuchs_reaction_step_3.svg|Corey–Fuchs reaction step 3 | |||
</gallery> | |||
Latest revision as of 05:01, 18 February 2025
Corey–Fuchs Reaction
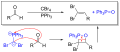
The Corey–Fuchs reaction, also known as the Corey–Fuchs alkyne synthesis, is a series of chemical reactions designed to synthesize alkynes from aldehydes. Named after its inventors, Elias James Corey and Philip L. Fuchs, this reaction is a pivotal method in organic chemistry for the formation of carbon-carbon bonds, enabling the transformation of simple aldehydes into more complex alkyne molecules. The Corey–Fuchs reaction is particularly valuable in the synthesis of natural products and pharmaceuticals.
Reaction Mechanism[edit]
The Corey–Fuchs reaction mechanism involves two key steps. Initially, an aldehyde is converted into a dibromide compound through the action of a phosphonium ylide, which is generated from triphenylphosphine (Ph3P) and carbon tetrabromide (CBr4). This step is followed by the elimination of hydrogen bromide (HBr) to form the alkyne. The overall process can be summarized as follows:
1. Formation of the phosphonium ylide from triphenylphosphine and carbon tetrabromide. 2. Reaction of the ylide with an aldehyde to produce a dibromide intermediate. 3. Elimination of HBr from the dibromide to yield the alkyne.
Applications[edit]
The Corey–Fuchs reaction has found widespread application in organic synthesis, particularly in the construction of complex molecular architectures. Its ability to efficiently convert aldehydes into alkynes makes it a valuable tool for the synthesis of natural products, pharmaceuticals, and materials science. Alkynes serve as versatile intermediates in organic synthesis, enabling further transformations into a variety of functional groups.
Limitations[edit]
Despite its utility, the Corey–Fuchs reaction has some limitations. The reaction conditions can sometimes lead to overreaction or the formation of unwanted by-products. Additionally, the use of toxic reagents such as carbon tetrabromide and triphenylphosphine may pose environmental and safety concerns. Researchers continue to explore modifications and alternatives to the Corey–Fuchs reaction to address these limitations.
See Also[edit]
References[edit]
<references/>
-
Corey–Fuchs reaction scheme
-
Corey–Fuchs reaction step 1
-
Corey–Fuchs reaction step 2
-
Corey–Fuchs reaction step 3