Molar heat capacity: Difference between revisions
CSV import |
CSV import |
||
| Line 44: | Line 44: | ||
{{stub}} | {{stub}} | ||
<gallery> | |||
File:Thermally_Agitated_Molecule.gif|Thermally agitated molecule | |||
File:DiatomicSpecHeat1.png|Diatomic specific heat 1 | |||
File:DiatomicSpecHeat2.png|Diatomic specific heat 2 | |||
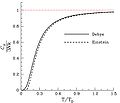
File:DebyeVSEinstein.jpg|Debye vs Einstein model | |||
</gallery> | |||
Latest revision as of 05:00, 18 February 2025
Molar Heat Capacity is a physical property of substances that describes the amount of heat required to change the temperature of one mole of a substance by one degree Celsius (or Kelvin). It is an important concept in thermodynamics, physical chemistry, and engineering because it helps scientists and engineers understand how substances absorb and transfer heat. The molar heat capacity is denoted by the symbol \(C_m\) and is measured in units of joules per mole per degree Celsius (\(J/(mol·°C)\)) or joules per mole per Kelvin (\(J/(mol·K)\)).
Definition[edit]
The molar heat capacity of a substance can be defined as the ratio of the amount of heat (\(q\)) added to or removed from a substance to the resulting temperature change (\(\Delta T\)) of one mole of the substance. Mathematically, it is expressed as:
\[C_m = \frac{q}{n\Delta T}\]
where:
- \(C_m\) is the molar heat capacity,
- \(q\) is the heat added or removed,
- \(n\) is the number of moles of the substance,
- \(\Delta T\) is the change in temperature.
Types[edit]
There are two main types of molar heat capacity:
- Specific Heat Capacity at Constant Volume (\(C_{m,V}\)): This is measured when the volume of the substance remains constant. It is particularly relevant in closed systems where no work is done.
- Specific Heat Capacity at Constant Pressure (\(C_{m,P}\)): This is measured when the pressure on the substance remains constant. It is more commonly used in practical scenarios, such as in atmospheric processes and chemical reactions happening at constant pressure.
Factors Affecting Molar Heat Capacity[edit]
Several factors can affect the molar heat capacity of a substance, including:
- Phase of Matter: Solid, liquid, and gaseous states of a substance have different molar heat capacities.
- Chemical Composition: The type and arrangement of atoms in a molecule can influence its ability to store and transfer heat.
- Temperature: For many substances, molar heat capacity changes with temperature.
- Pressure: Although the effect of pressure on molar heat capacity is generally smaller than that of temperature, it can still be significant under extreme conditions.
Applications[edit]
Molar heat capacity plays a crucial role in various scientific and engineering fields. It is essential in:
- Designing thermal management systems for electronics and machinery,
- Developing efficient energy storage and transfer systems,
- Understanding and predicting climate change through the study of atmospheric gases,
- Analyzing and improving chemical reaction processes in terms of energy efficiency.
Measurement[edit]
The molar heat capacity can be measured using calorimetry, a technique that involves measuring the heat exchanged during a physical or chemical process. Different types of calorimeters are used depending on the conditions (constant pressure or constant volume) under which the heat capacity is to be measured.