Boron compounds: Difference between revisions
CSV import |
CSV import |
||
| Line 31: | Line 31: | ||
[[Category:Boron compounds]] | [[Category:Boron compounds]] | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
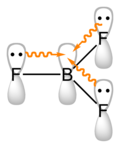
File:Boron-trifluoride-pi-bonding-2D.png|Boron trifluoride pi bonding | |||
File:Deltahedral-borane-cluster-array-numbered-3D-bs-17.png|Deltahedral borane cluster | |||
File:Magnesium-diboride-3D-balls.png|Magnesium diboride structure | |||
</gallery> | |||
Latest revision as of 04:07, 18 February 2025
Boron Compounds are chemical compounds that contain boron, a semi-metallic element with the atomic number 5. Boron compounds play a crucial role in many chemical and industrial applications, including the production of glass, ceramics, and detergents. They are also used in the field of medicine and agriculture.
Overview[edit]
Boron compounds are characterized by their high melting points and hardness. They are typically colorless, but can also be found in various colors depending on the specific compound. The most common boron compounds include boric acid, boron carbide, and boron nitride.
Boric Acid[edit]
Boric acid, also known as hydrogen borate, is a weak, monobasic Lewis acid of boron. It has antiseptic, antifungal, and antiviral properties, and is used in medical applications, as an insecticide, and in the manufacture of heat-resistant glass and porcelain enamels.
Boron Carbide[edit]
Boron carbide is a hard boron-carbon ceramic and covalent material used in tank armor, bulletproof vests, and numerous industrial applications. It is one of the hardest known materials, behind cubic boron nitride and diamond.
Boron Nitride[edit]
Boron nitride is a heat and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice.
Applications[edit]
Boron compounds have a wide range of applications. In addition to their use in the production of glass and ceramics, they are also used in the manufacture of detergents and as flame retardants. In the field of medicine, boric acid is used as an antiseptic, and boron compounds are being studied for use in cancer treatment. In agriculture, boron compounds are used as micronutrients in fertilizers.
See Also[edit]
-
Boron trifluoride pi bonding
-
Deltahedral borane cluster
-
Magnesium diboride structure