Meisenheimer complex: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 26: | Line 26: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:MeisenheimerClean.svg|Meisenheimer complex structure | |||
File:MeisenHeimerWhelandComplex.png|Meisenheimer-Wheland complex | |||
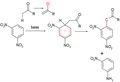
File:ZimmermannReaction.png|Zimmermann reaction | |||
</gallery> | |||
Latest revision as of 02:00, 18 February 2025
Meisenheimer Complex
The Meisenheimer complex or Meisenheimer-Jackson complex is a key intermediate in organic chemistry, particularly in the realm of nucleophilic aromatic substitution reactions. This complex is named after the German chemist Jakob Meisenheimer, who first proposed its existence in the early 20th century. The Meisenheimer complex represents a pivotal moment in the reaction mechanism where an aromatic ring, typically stable and resistant to direct nucleophilic attack, temporarily accommodates a nucleophile, leading to the addition of new substituents or the rearrangement of existing ones.
Formation[edit]
The formation of a Meisenheimer complex occurs when a nucleophile attacks an electron-deficient aromatic compound, such as those containing nitro, sulfonyl, or cyano groups. These electron-withdrawing groups activate the aromatic ring towards nucleophilic attack by increasing the positive character of the carbon atoms. Upon the nucleophile's approach, the aromatic system's pi electrons are temporarily disrupted, leading to the formation of a cyclohexadienyl cation-like structure. This intermediate is the Meisenheimer complex, characterized by its non-aromatic, charged nature, which is stabilized by the electron-withdrawing groups attached to the ring.
Structure and Stability[edit]
The structure of a Meisenheimer complex involves a sigma bond between the nucleophile and the target carbon atom of the aromatic ring, with the charge being delocalized over the ring and the electron-withdrawing groups. The stability of this complex is highly dependent on the nature of the substituents attached to the aromatic ring. Electron-withdrawing groups that are capable of delocalizing the charge effectively will enhance the stability of the complex, facilitating the subsequent steps of the reaction mechanism.
Significance in Organic Chemistry[edit]
The Meisenheimer complex is significant in organic chemistry for several reasons. Firstly, it provides a mechanistic understanding of how nucleophilic aromatic substitution reactions proceed, offering insights into the reactivity and selectivity of these reactions. Secondly, the existence of this complex allows for the functionalization of aromatic compounds in ways that would be challenging through direct electrophilic aromatic substitution. This has broad implications for the synthesis of pharmaceuticals, agrochemicals, and materials science.
Examples[edit]
One of the most studied reactions involving the Meisenheimer complex is the reaction of 1,3,5-trinitrobenzene with various nucleophiles. In this reaction, the nucleophile attacks one of the nitro-substituted carbon atoms, forming a Meisenheimer complex that can further react to substitute a nitro group, leading to a variety of substitution products.
See Also[edit]
-
Meisenheimer complex structure
-
Meisenheimer-Wheland complex
-
Zimmermann reaction