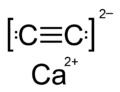Calcium carbide: Difference between revisions
CSV import |
CSV import |
||
| Line 44: | Line 44: | ||
[[Category:Carbides]] | [[Category:Carbides]] | ||
[[Category:Industrial gases]] | [[Category:Industrial gases]] | ||
<gallery> | |||
File:Calcium_carbide_formula.png|Chemical formula of calcium carbide | |||
File:Karbid_vápenat_.JPG|Calcium carbide in solid form | |||
File:Carbide_lamp_lit.jpg|A lit carbide lamp | |||
</gallery> | |||
Revision as of 01:55, 18 February 2025
Chemical compound used in the production of acetylene gas
Calcium Carbide

Calcium carbide is a chemical compound with the chemical formula CaC_. It is primarily used in the production of acetylene gas, which is utilized in various industrial applications, including welding and as a precursor to other chemicals.
Chemical Properties
Calcium carbide is a grayish-black solid at room temperature. It reacts with water to produce acetylene gas and calcium hydroxide according to the following chemical equation:
- CaC_ + 2H_O _ C_H_ + Ca(OH)_
This reaction is highly exothermic and is the basis for its use in carbide lamps and other applications.
Production
Calcium carbide is produced industrially in an electric arc furnace from a mixture of lime (CaO) and coke at approximately 2,200 °C (3,990 °F). The reaction is as follows:
- CaO + 3C _ CaC_ + CO
The resulting calcium carbide is then cooled and crushed to the desired size.
Applications
Acetylene Production
The primary use of calcium carbide is in the production of acetylene gas. Acetylene is used as a fuel and a chemical building block. It is particularly important in the welding industry due to its high flame temperature.
Carbide Lamps

Calcium carbide is used in carbide lamps, which were once common in mining and caving. These lamps produce light by the combustion of acetylene gas generated from the reaction of calcium carbide with water.
Chemical Synthesis
Calcium carbide is also used in the synthesis of various organic chemicals. It serves as a precursor to acetylene derivatives and other chemicals in the chemical industry.
Safety and Handling
Calcium carbide must be handled with care due to its reactivity with water and the flammability of acetylene gas. Proper storage and handling procedures are essential to prevent accidental ignition or explosion.
Related pages
-
Chemical formula of calcium carbide
-
Calcium carbide in solid form
-
A lit carbide lamp