Protein O-GlcNAcase: Difference between revisions
CSV import |
CSV import |
||
| Line 21: | Line 21: | ||
[[Category:Protein metabolism]] | [[Category:Protein metabolism]] | ||
{{Medicine-stub}} | {{Medicine-stub}} | ||
<gallery> | |||
File:Cartoon_Image_of_OGA.jpg|Cartoon image of OGA | |||
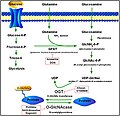
File:Metabolic_pathway_for_OGA.jpg|Metabolic pathway for OGA | |||
File:F3_Inhibitor.jpg|F3 Inhibitor | |||
</gallery> | |||
Latest revision as of 01:40, 18 February 2025
Protein O-GlcNAcase (OGA) is an enzyme that plays a critical role in the post-translational modification of proteins through the removal of O-linked N-acetylglucosamine (O-GlcNAc) residues. This modification is a dynamic and reversible process that is essential for the regulation of numerous cellular functions, including signal transduction, transcription, and protein degradation. O-GlcNAcylation, the addition of O-GlcNAc to serine or threonine residues on nuclear and cytoplasmic proteins, is countered by the action of OGA, making it a key player in cellular homeostasis and signaling.
Function[edit]
The primary function of OGA is to hydrolyze the O-GlcNAc modification on proteins, a process that is crucial for the modulation of protein function and the regulation of cellular signaling pathways. By removing O-GlcNAc groups, OGA influences various cellular processes such as nutrient sensing, stress response, and the cell cycle. The balance between O-GlcNAc addition by O-GlcNAc transferase (OGT) and removal by OGA is vital for the proper functioning of cellular mechanisms and the prevention of disease.
Structure[edit]
Protein O-GlcNAcase is a multifunctional enzyme that exists in multiple isoforms, which are generated through alternative splicing. The enzyme contains distinct domains responsible for its catalytic activity and substrate specificity. The catalytic domain of OGA is highly conserved across different species, indicating the evolutionary importance of its function in cellular physiology.
Clinical Significance[edit]
Alterations in the activity or expression of OGA have been implicated in the pathogenesis of several diseases, including diabetes, Alzheimer's disease, and cancer. In diabetes, aberrant O-GlcNAcylation can affect insulin signaling and glucose metabolism. In Alzheimer's disease, abnormal O-GlcNAcylation of tau protein, mediated by imbalances in OGA and OGT activities, contributes to tau pathology and neurodegeneration. Furthermore, dysregulation of O-GlcNAcylation has been associated with the development and progression of cancer, highlighting the potential of OGA as a therapeutic target.
Therapeutic Potential[edit]
Given its role in various diseases, OGA has emerged as a potential therapeutic target. Inhibitors of OGA have been developed and are being explored for their therapeutic potential in conditions such as neurodegenerative diseases and cancer. By modulating O-GlcNAcylation levels, OGA inhibitors could offer a novel approach to the treatment of diseases associated with dysregulated O-GlcNAcylation.
Research Directions[edit]
Research on OGA continues to uncover its complex roles in cellular physiology and disease. Future studies are aimed at elucidating the detailed mechanisms by which OGA regulates specific cellular pathways and how its activity is modulated in different physiological and pathological contexts. Understanding the intricate balance between OGA and OGT activities will be crucial for the development of targeted therapies aimed at modulating O-GlcNAcylation for disease treatment.
-
Cartoon image of OGA
-
Metabolic pathway for OGA
-
F3 Inhibitor