Fujimoto–Belleau reaction: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 24: | Line 24: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
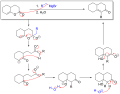
File:Fujimoto-Belleau_Reaction_Scheme.png|Fujimoto–Belleau reaction scheme | |||
File:Fujimoto-Belleau_reaction_mechanism.svg|Fujimoto–Belleau reaction mechanism | |||
</gallery> | |||
Latest revision as of 01:13, 18 February 2025
Fujimoto–Belleau reaction is a chemical reaction named after the scientists who first described it, Hiroshi Fujimoto and Bernard Belleau, in the mid-20th century. This reaction involves the conversion of certain alkyl halides into amines through a process known as nucleophilic substitution. Specifically, the Fujimoto–Belleau reaction is notable for its application in the synthesis of tertiary amines from primary amines, using a secondary amine as a nucleophile. This reaction is an important method in the field of organic chemistry, particularly in the synthesis of pharmaceuticals and other nitrogen-containing compounds.
Mechanism[edit]
The mechanism of the Fujimoto–Belleau reaction involves several key steps. Initially, the primary amine reacts with a suitable alkylating agent, typically an alkyl halide, to form a secondary amine. This intermediate then undergoes further alkylation to produce a tertiary amine. The reaction conditions, such as the choice of solvent and temperature, play a crucial role in determining the yield and purity of the final product. The use of excess alkylating agent can drive the reaction towards the formation of the desired tertiary amine.
Applications[edit]
The Fujimoto–Belleau reaction has found widespread application in the synthesis of various organic compounds, particularly those with medicinal properties. Tertiary amines are a common structural motif in many pharmaceuticals, and the ability to synthesize these compounds efficiently is of great importance to the pharmaceutical industry. Additionally, this reaction is used in the preparation of agrochemicals, dyes, and other industrial chemicals.
Advantages and Limitations[edit]
One of the main advantages of the Fujimoto–Belleau reaction is its simplicity and efficiency in synthesizing tertiary amines. However, the reaction also has some limitations. The use of alkyl halides as alkylating agents can lead to the formation of quaternary ammonium salts as side products, which can complicate the purification process. Moreover, the reaction conditions need to be carefully controlled to avoid over-alkylation and the formation of unwanted by-products.
See Also[edit]
References[edit]
<references/>
-
Fujimoto–Belleau reaction scheme
-
Fujimoto–Belleau reaction mechanism