KMT2A: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 26: | Line 26: | ||
{{Gene-stub}} | {{Gene-stub}} | ||
{{Medicine-stub}} | {{Medicine-stub}} | ||
== KMT2A == | |||
<gallery> | |||
File:KMT2A.jpg|KMT2A protein structure | |||
File:9aaTADs_in_the_E_protein_family.jpg|9aaTADs in the E protein family | |||
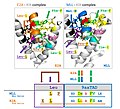
File:Piskacek_Figures_v9b.jpg|Piskacek Figures version 9b | |||
File:Gray626.png|Gray's Anatomy illustration 626 | |||
</gallery> | |||
Revision as of 00:55, 18 February 2025
KMT2A (Lysine Methyltransferase 2A), formerly known as MLL (Mixed-Lineage Leukemia), is a gene located on chromosome 11q23 that plays a critical role in the regulation of gene expression through histone modification. This gene is particularly significant in the context of developmental processes and hematopoiesis. Mutations and translocations involving the KMT2A gene are closely associated with various types of leukemia and other hematologic malignancies.
Function
The KMT2A gene encodes a protein that is a member of the SET domain methyltransferase family. This protein functions as a histone methyltransferase that specifically mono-, di-, and trimethylates 'Lys-4' of histone H3. The methylation of histone H3 at Lys-4 (H3K4me) is a key epigenetic modification associated with gene activation. By modifying chromatin structure, KMT2A plays a pivotal role in the transcriptional regulation of genes involved in embryonic development and hematopoiesis.
Clinical Significance
Alterations in the KMT2A gene, including point mutations, partial tandem duplications, and chromosomal translocations, are implicated in the pathogenesis of several hematological malignancies. The most common alteration is the KMT2A rearrangement, which occurs through translocations with various partner genes. These translocations result in the production of fusion proteins that possess altered epigenetic regulatory functions, contributing to leukemogenesis.
Leukemia
KMT2A rearrangements are most frequently observed in acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). In particular, the t(4;11)(q21;q23) translocation involving the KMT2A and AFF1 genes is a hallmark of infant ALL. These genetic alterations disrupt normal hematopoiesis and lead to the uncontrolled proliferation of leukemic cells.
Diagnosis and Prognosis
The detection of KMT2A rearrangements is crucial for the diagnosis and risk stratification of leukemia. Various molecular biology techniques, including fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), and next-generation sequencing (NGS), are employed to identify KMT2A gene alterations. Patients with KMT2A-rearranged leukemia often have a poor prognosis and require aggressive treatment strategies.
Treatment
The treatment of KMT2A-rearranged leukemia typically involves intensive chemotherapy, targeted therapy, and, in some cases, hematopoietic stem cell transplantation. Recent advances in targeted therapy have led to the development of small molecule inhibitors that specifically target the enzymatic activity of KMT2A fusion proteins, offering a promising therapeutic approach for patients with this genetic alteration.
Research Directions
Ongoing research aims to better understand the molecular mechanisms by which KMT2A alterations contribute to leukemogenesis and to develop more effective and less toxic therapeutic strategies. The identification of novel therapeutic targets and the design of targeted therapies hold the potential to improve outcomes for patients with KMT2A-rearranged leukemia.
KMT2A
-
KMT2A protein structure
-
9aaTADs in the E protein family
-
Piskacek Figures version 9b
-
Gray's Anatomy illustration 626