Telcagepant: Difference between revisions
CSV import |
CSV import |
||
| Line 29: | Line 29: | ||
<gallery> | <gallery> | ||
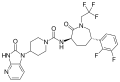
File:Telcagepant_structure.svg|Structure of Telcagepant | File:Telcagepant_structure.svg|Structure of Telcagepant | ||
File:Telcagepant-3D-balls.png|3D ball model of Telcagepant | |||
</gallery> | |||
== Telcagepant == | |||
<gallery> | |||
File:Telcagepant_structure.svg|Chemical structure of Telcagepant | |||
File:Telcagepant-3D-balls.png|3D ball model of Telcagepant | File:Telcagepant-3D-balls.png|3D ball model of Telcagepant | ||
</gallery> | </gallery> | ||
Revision as of 00:41, 18 February 2025
Telcagepant is a drug that was under development for the acute treatment of migraines. It belongs to the class of drugs known as calcitonin gene-related peptide receptor (CGRP receptor) antagonists. Telcagepant was being developed by Merck & Co., but its development was discontinued in 2011 due to concerns about its potential to cause liver damage.
History
Telcagepant was first synthesized by Merck & Co. in the early 2000s as part of their research into CGRP receptor antagonists. Early clinical trials showed promise, with the drug demonstrating efficacy in the treatment of acute migraines. However, in 2011, Merck announced that they were discontinuing the development of Telcagepant due to concerns about its potential to cause liver damage.
Mechanism of Action
Telcagepant works by blocking the CGRP receptor. CGRP is a neuropeptide that plays a key role in the pathophysiology of migraines. By blocking this receptor, Telcagepant prevents the vasodilation and inflammation that are associated with migraines.
Clinical Trials
Several clinical trials were conducted to assess the efficacy and safety of Telcagepant. These trials showed that the drug was effective in treating acute migraines, with a similar efficacy to triptans, the standard treatment for migraines. However, some patients in the trials experienced elevated liver enzymes, a potential sign of liver damage. This led to the discontinuation of the drug's development.
Potential Side Effects
The most common side effects observed in clinical trials of Telcagepant were nausea, dizziness, and dry mouth. However, the potential for liver damage was the most significant concern, leading to the discontinuation of the drug's development.
See Also
-
Structure of Telcagepant
-
3D ball model of Telcagepant
Telcagepant
-
Chemical structure of Telcagepant
-
3D ball model of Telcagepant