Nimustine: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 27: | Line 27: | ||
[[Category:Alkylating agents]] | [[Category:Alkylating agents]] | ||
{{Chemotherapy-stub}} | {{Chemotherapy-stub}} | ||
== Nimustine == | |||
<gallery> | |||
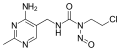
File:Nimustine.svg|Chemical structure of Nimustine | |||
File:Nimustine_3D_spacefill.png|3D space-filling model of Nimustine | |||
</gallery> | |||
Latest revision as of 02:12, 17 February 2025
Nimustine (also known as ACNU) is a nitrosourea compound used in chemotherapy. It is classified as an alkylating agent, which means it works by damaging the DNA of cancer cells to prevent them from dividing and growing.
History[edit]
Nimustine was first synthesized in the 1970s as part of a series of nitrosourea compounds developed for their potential anti-cancer properties. It has since been used in the treatment of various types of cancer, including brain tumors, lung cancer, and leukemia.
Mechanism of Action[edit]
As an alkylating agent, nimustine works by transferring an alkyl group to the DNA molecule. This causes DNA damage, which prevents the cancer cells from dividing and growing. Nimustine is also believed to inhibit the enzyme O6-methylguanine-DNA methyltransferase, which is involved in DNA repair.
Side Effects[edit]
Like all chemotherapy drugs, nimustine can cause side effects. These can include nausea, vomiting, hair loss, and bone marrow suppression. More serious side effects can include neurotoxicity and pulmonary fibrosis.
Pharmacokinetics[edit]
Nimustine is usually administered intravenously. It is metabolized in the liver and excreted in the urine. The half-life of nimustine is approximately 2 hours.
See Also[edit]
This Chemotherapy related article is a stub. You can help WikiMD by expanding it.
Nimustine[edit]
-
Chemical structure of Nimustine
-
3D space-filling model of Nimustine