8,11-Dihydroxytetrahydrocannabinol: Difference between revisions
CSV import |
CSV import |
||
| Line 26: | Line 26: | ||
[[Category:Cannabinoids]] | [[Category:Cannabinoids]] | ||
[[Category:Metabolites]] | [[Category:Metabolites]] | ||
== 8,11-Dihydroxytetrahydrocannabinol == | |||
<gallery> | |||
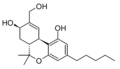
File:8B-11-diOH-THC_structure.png|Structure of 8,11-Dihydroxytetrahydrocannabinol | |||
</gallery> | |||
Latest revision as of 22:09, 16 February 2025
A metabolite of tetrahydrocannabinol
8,11-Dihydroxytetrahydrocannabinol (8,11-diOH-THC) is a metabolite of tetrahydrocannabinol (THC), the primary psychoactive component of cannabis. This compound is formed in the body after the consumption of THC and is part of the metabolic pathway that processes cannabinoids.
Chemical structure[edit]
8,11-Dihydroxytetrahydrocannabinol is characterized by the presence of two hydroxyl groups at the 8th and 11th positions of the tetrahydrocannabinol molecule. This structural modification is significant as it alters the compound's pharmacokinetics and pharmacodynamics compared to its parent compound, THC.

Metabolism[edit]
The metabolism of THC involves several steps, primarily occurring in the liver. The initial phase involves the conversion of THC to 11-hydroxy-THC (11-OH-THC) by the enzyme cytochrome P450 2C9. Subsequently, 11-OH-THC is further metabolized to 8,11-dihydroxytetrahydrocannabinol. This process is part of the body's mechanism to increase the compound's water solubility for easier excretion.
Pharmacological effects[edit]
While 8,11-dihydroxytetrahydrocannabinol is a metabolite of THC, its pharmacological effects are not as well-studied. However, it is known that hydroxylation of THC can influence the compound's psychoactive properties. The presence of additional hydroxyl groups may affect the compound's ability to cross the blood-brain barrier and interact with cannabinoid receptors.
Excretion[edit]
8,11-Dihydroxytetrahydrocannabinol, like other THC metabolites, is eventually excreted from the body through urine and feces. The increased polarity of the compound due to hydroxylation facilitates its elimination.
Related pages[edit]
8,11-Dihydroxytetrahydrocannabinol[edit]
-
Structure of 8,11-Dihydroxytetrahydrocannabinol