Tribromofluoromethane: Difference between revisions
CSV import |
CSV import |
||
| Line 52: | Line 52: | ||
[[Category:Fire suppression agents]] | [[Category:Fire suppression agents]] | ||
[[Category:Ozone depletion]] | [[Category:Ozone depletion]] | ||
<gallery> | |||
File:Fluorotribromomethane_Formula_V1.svg | |||
</gallery> | |||
Latest revision as of 22:01, 16 February 2025
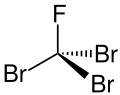
A halomethane compound with the formula CBr_F
| Chemical Compound | |
|---|---|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider ID | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Properties | |
| Chemical Formula | |
| Molar Mass | |
| Appearance | |
| Density | |
| Melting Point | |
| Boiling Point | |
| Hazards | |
| GHS Pictograms | [[File:|50px]] |
| GHS Signal Word | |
| GHS Hazard Statements | |
| NFPA 704 | [[File:|50px]] |
| References | |
Tribromofluoromethane, also known as fluorotribromomethane, is a halomethane compound with the chemical formula CBr_F. It is a colorless liquid at room temperature and is primarily used in fire extinguishing systems.
Properties[edit]
Tribromofluoromethane is characterized by its high density and low boiling point. It is a non-flammable compound, which makes it suitable for use in fire suppression. The compound is stable under normal conditions but can decompose when exposed to high temperatures, releasing toxic gases such as hydrogen bromide and hydrogen fluoride.
Synthesis[edit]
Tribromofluoromethane can be synthesized through the halogenation of methane using bromine and fluorine. The process involves the substitution of hydrogen atoms in methane with bromine and fluorine atoms, resulting in the formation of CBr_F.
Applications[edit]
The primary application of tribromofluoromethane is in fire extinguishing systems, particularly in environments where water-based extinguishers are unsuitable. It is used in Halon fire suppression systems, which are effective in extinguishing fires without leaving residue. However, due to environmental concerns, the use of halons, including tribromofluoromethane, has been restricted under the Montreal Protocol due to their ozone-depleting potential.
Environmental Impact[edit]
Tribromofluoromethane is classified as an ozone-depleting substance. Its release into the atmosphere contributes to the depletion of the ozone layer, which protects the Earth from harmful ultraviolet radiation. As a result, its production and use have been phased out in many countries in favor of more environmentally friendly alternatives.
Safety[edit]
Handling tribromofluoromethane requires caution due to its potential to release toxic gases upon decomposition. Proper ventilation and protective equipment are recommended when working with this compound. In case of exposure, immediate medical attention is advised.