Trichloroacetonitrile: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} | |||
{{Chembox | |||
| verifiedfields = changed | |||
| verifiedrevid = 477239123 | |||
| Name = Trichloroacetonitrile | |||
| ImageFile = Trichloroacetonitrile_Structure_V.1.svg | |||
| ImageSize = 150px | |||
| IUPACName = Trichloroacetonitrile | |||
| OtherNames = Trichloroacetonitrile | |||
}} | |||
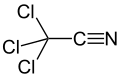
'''Trichloroacetonitrile''' is a [[nitrile]] with the chemical formula CCl_CN. It is a colorless liquid that is used as an intermediate in the synthesis of various chemical compounds. | |||
Trichloroacetonitrile is a [[nitrile]] | |||
==Synthesis== | ==Synthesis== | ||
Trichloroacetonitrile can be synthesized | Trichloroacetonitrile can be synthesized through several methods. One common method involves the reaction of [[trichloroacetamide]] with [[phosphorus pentoxide]]: | ||
== | [[File:TCAN_via_Trichloracetamid.svg|thumb|center|500px|Synthesis of trichloroacetonitrile from trichloroacetamide]] | ||
Another method involves the chlorination of [[acetonitrile]]: | |||
[[File:TCAN_via_Acetonitril.svg|thumb|center|500px|Synthesis of trichloroacetonitrile from acetonitrile]] | |||
==Applications== | |||
Trichloroacetonitrile is used as a reagent in organic synthesis. It is particularly useful in the preparation of [[imidates]] and [[glycosyl donors]]. | |||
===Imidate Formation=== | |||
Trichloroacetonitrile reacts with alcohols to form trichloroacetimidates, which are useful intermediates in organic synthesis: | |||
[[File:Trichloracetimidat-Bildung.svg|thumb|center|500px|Formation of trichloroacetimidate]] | |||
===Glycosylation Reactions=== | |||
Trichloroacetonitrile is used in the synthesis of glycosyl trichloroacetimidates, which are important in the formation of glycosidic bonds: | |||
[[File:Alpha-Glycosyltrichloracetimidat.svg|thumb|center|500px|Synthesis of glycosyl trichloroacetimidate]] | |||
==Safety== | ==Safety== | ||
Trichloroacetonitrile is a hazardous | Trichloroacetonitrile is a hazardous chemical and should be handled with care. It is toxic if inhaled or ingested and can cause skin and eye irritation. | ||
== | ==Related pages== | ||
* [[Nitrile]] | * [[Nitrile]] | ||
* [[Chlorination]] | |||
* [[Organic synthesis]] | |||
==References== | |||
{{Reflist}} | |||
==Gallery== | |||
<gallery> | |||
File:Trichloroacetonitrile_Structure_V.1.svg|Structure of trichloroacetonitrile | |||
File:TCAN_via_Trichloracetamid.svg|Synthesis from trichloroacetamide | |||
File:TCAN_via_Acetonitril.svg|Synthesis from acetonitrile | |||
File:Trichloroacetonitrile_dimensions.svg|Molecular dimensions | |||
File:Allylchloride_via_Allylalkohole.svg|Related synthesis | |||
File:2-Chlorpyridin_via_TCAN.svg|Synthesis of 2-chloropyridine | |||
File:Hydroxyketone_via_TCAN.svg|Synthesis of hydroxyketone | |||
File:Trichloracetimidat-Bildung.svg|Formation of trichloroacetimidate | |||
File:Alpha-Glycosyltrichloracetimidat.svg|Glycosyl trichloroacetimidate | |||
File:Octaacetyl-Trehalose.svg|Octaacetyl trehalose | |||
File:Thiogalactose-Synthese.svg|Thiogalactose synthesis | |||
</gallery> | |||
[[Category:Nitriles]] | [[Category:Nitriles]] | ||
[[Category: | [[Category:Organochlorides]] | ||
Revision as of 00:37, 10 February 2025
Chemical compound
| Chemical Compound | |
|---|---|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider ID | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Properties | |
| Chemical Formula | |
| Molar Mass | |
| Appearance | |
| Density | |
| Melting Point | |
| Boiling Point | |
| Hazards | |
| GHS Pictograms | [[File:|50px]] |
| GHS Signal Word | |
| GHS Hazard Statements | |
| NFPA 704 | [[File:|50px]] |
| References | |
Trichloroacetonitrile is a nitrile with the chemical formula CCl_CN. It is a colorless liquid that is used as an intermediate in the synthesis of various chemical compounds.
Synthesis
Trichloroacetonitrile can be synthesized through several methods. One common method involves the reaction of trichloroacetamide with phosphorus pentoxide:

Another method involves the chlorination of acetonitrile:

Applications
Trichloroacetonitrile is used as a reagent in organic synthesis. It is particularly useful in the preparation of imidates and glycosyl donors.
Imidate Formation
Trichloroacetonitrile reacts with alcohols to form trichloroacetimidates, which are useful intermediates in organic synthesis:

Glycosylation Reactions
Trichloroacetonitrile is used in the synthesis of glycosyl trichloroacetimidates, which are important in the formation of glycosidic bonds:

Safety
Trichloroacetonitrile is a hazardous chemical and should be handled with care. It is toxic if inhaled or ingested and can cause skin and eye irritation.
Related pages
References
<references group="" responsive="1"></references>
Gallery
-
Structure of trichloroacetonitrile
-
Synthesis from trichloroacetamide
-
Synthesis from acetonitrile
-
Molecular dimensions
-
Related synthesis
-
Synthesis of 2-chloropyridine
-
Synthesis of hydroxyketone
-
Formation of trichloroacetimidate
-
Glycosyl trichloroacetimidate
-
Octaacetyl trehalose
-
Thiogalactose synthesis