Japp–Klingemann reaction: Difference between revisions
CSV import |
CSV import |
||
| Line 1: | Line 1: | ||
= Japp–Klingemann reaction = | |||
The '''Japp–Klingemann reaction''' is a chemical reaction used in organic chemistry to synthesize [[hydrazone]]s from [[aryl diazonium salt]]s and [[ | The '''Japp–Klingemann reaction''' is a chemical reaction used in organic chemistry to synthesize [[hydrazone]]s from [[aryl diazonium salt]]s and [[β-keto acid]]s or their esters. This reaction is named after the chemists Francis Robert Japp and Felix Klingemann, who first reported it in 1887. | ||
==Reaction mechanism== | == Reaction mechanism == | ||
The Japp–Klingemann reaction involves the coupling of an aryl diazonium salt with a β-keto ester or β-keto acid to form a hydrazone intermediate. This intermediate can then undergo further transformations, such as cyclization, to yield various heterocyclic compounds. | |||
[[File:Japp- | [[File:Japp-Klingemann_Reaction_Scheme.png|thumb|right|400px|General scheme of the Japp–Klingemann reaction.]] | ||
The mechanism begins with the formation of the aryl diazonium salt from an aniline derivative. This diazonium salt then reacts with the β-keto compound to form the hydrazone. The reaction is typically carried out in an acidic medium to facilitate the diazonium salt formation and subsequent coupling. | |||
The | |||
== | == Applications == | ||
The Japp–Klingemann reaction is particularly useful in the synthesis of [[pyrazole]] derivatives, which are important in medicinal chemistry and agrochemicals. The reaction can be used to construct complex heterocyclic structures that are otherwise difficult to synthesize. | |||
== | === Synthesis of pyrazoles === | ||
The Japp–Klingemann reaction | |||
One of the primary applications of the Japp–Klingemann reaction is in the synthesis of pyrazoles. The hydrazone intermediate can undergo cyclization to form the pyrazole ring. | |||
[[File:Arylpyrazole_via_Japp-Klingemann.png|thumb|left|400px|Synthesis of arylpyrazole via the Japp–Klingemann reaction.]] | |||
=== Combination with Fischer indole synthesis === | |||
The Japp–Klingemann reaction can be combined with the [[Fischer indole synthesis]] to produce indole derivatives. This combination allows for the construction of complex indole structures from simple starting materials. | |||
[[File:Japp-Klingemann_Fischer_Indole_Combination.png|thumb|right|400px|Combination of Japp–Klingemann reaction with Fischer indole synthesis.]] | |||
== Mechanistic details == | |||
The detailed mechanism of the Japp–Klingemann reaction involves several key steps: | |||
1. Formation of the aryl diazonium salt from an aniline derivative. | |||
2. Nucleophilic attack of the β-keto compound on the diazonium salt, forming the hydrazone. | |||
3. Possible cyclization of the hydrazone to form heterocyclic compounds. | |||
[[File:Japp-Klingemann_Ester_Mechanism.png|thumb|left|400px|Mechanism of the Japp–Klingemann reaction with esters.]] | |||
== Example: Synthesis of Pyraclofos == | |||
The Japp–Klingemann reaction can be used in the synthesis of complex molecules such as [[Pyraclofos]], an organophosphate insecticide. The reaction provides a route to the hydrazone intermediate, which can be further transformed into the desired product. | |||
[[File:Pyraclofos_Synthesis.svg|thumb|right|400px|Synthesis of Pyraclofos using the Japp–Klingemann reaction.]] | |||
== Related pages == | |||
* [[Diazonium compound]] | * [[Diazonium compound]] | ||
* [[Hydrazone]] | * [[Hydrazone]] | ||
* [[ | * [[Pyrazole]] | ||
* [[Fischer indole synthesis]] | |||
* [[ | |||
[[Category:Organic reactions]] | [[Category:Organic reactions]] | ||
[[Category:Name reactions]] | [[Category:Name reactions]] | ||
Revision as of 14:24, 21 February 2025
Japp–Klingemann reaction
The Japp–Klingemann reaction is a chemical reaction used in organic chemistry to synthesize hydrazones from aryl diazonium salts and β-keto acids or their esters. This reaction is named after the chemists Francis Robert Japp and Felix Klingemann, who first reported it in 1887.
Reaction mechanism
The Japp–Klingemann reaction involves the coupling of an aryl diazonium salt with a β-keto ester or β-keto acid to form a hydrazone intermediate. This intermediate can then undergo further transformations, such as cyclization, to yield various heterocyclic compounds.
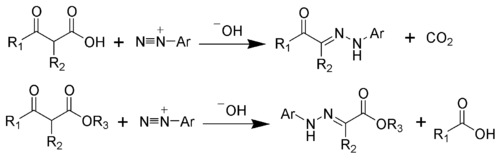
The mechanism begins with the formation of the aryl diazonium salt from an aniline derivative. This diazonium salt then reacts with the β-keto compound to form the hydrazone. The reaction is typically carried out in an acidic medium to facilitate the diazonium salt formation and subsequent coupling.
Applications
The Japp–Klingemann reaction is particularly useful in the synthesis of pyrazole derivatives, which are important in medicinal chemistry and agrochemicals. The reaction can be used to construct complex heterocyclic structures that are otherwise difficult to synthesize.
Synthesis of pyrazoles
One of the primary applications of the Japp–Klingemann reaction is in the synthesis of pyrazoles. The hydrazone intermediate can undergo cyclization to form the pyrazole ring.

Combination with Fischer indole synthesis
The Japp–Klingemann reaction can be combined with the Fischer indole synthesis to produce indole derivatives. This combination allows for the construction of complex indole structures from simple starting materials.

Mechanistic details
The detailed mechanism of the Japp–Klingemann reaction involves several key steps:
1. Formation of the aryl diazonium salt from an aniline derivative. 2. Nucleophilic attack of the β-keto compound on the diazonium salt, forming the hydrazone. 3. Possible cyclization of the hydrazone to form heterocyclic compounds.

Example: Synthesis of Pyraclofos
The Japp–Klingemann reaction can be used in the synthesis of complex molecules such as Pyraclofos, an organophosphate insecticide. The reaction provides a route to the hydrazone intermediate, which can be further transformed into the desired product.