Tropolone: Difference between revisions
CSV import |
CSV import |
||
| Line 42: | Line 42: | ||
[[Category:Aromatic compounds]] | [[Category:Aromatic compounds]] | ||
[[Category:Seven-membered rings]] | [[Category:Seven-membered rings]] | ||
<gallery> | |||
File:TropoloneGenSynth.png|General synthesis of Tropolone | |||
File:OS_tropolone_from_CpH.svg|Synthesis of Tropolone from Cyclopentadiene | |||
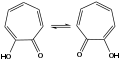
File:TropoloneTaut.svg|Tautomerism of Tropolone | |||
File:Tropolone.png|Structure of Tropolone | |||
File:Gamma-thujaplicin.png|Structure of Gamma-thujaplicin | |||
File:Stipitatic_acid.svg|Structure of Stipitatic acid | |||
File:Colchicin.svg|Structure of Colchicin | |||
</gallery> | |||
Latest revision as of 11:07, 18 February 2025
Tropolone[edit]



Tropolone is a chemical compound with the molecular formula C_H_O_. It is a member of the class of compounds known as tropones, which are characterized by a seven-membered aromatic ring containing a carbonyl group. Tropolone is notable for its unique structure and properties, which have been the subject of extensive research in organic chemistry.
Structure and Properties[edit]
Tropolone is a cyclic ketone with a seven-membered ring, which is unusual compared to the more common six-membered aromatic rings. The presence of the carbonyl group in the ring contributes to its aromaticity, and the compound exhibits tautomerism, existing in equilibrium between keto and enol forms. This tautomeric behavior is depicted in the accompanying diagram.
Synthesis[edit]
Tropolone can be synthesized through several methods. One common approach involves the oxidation of cycloheptatriene derivatives. The diagram shows a typical synthetic route starting from cycloheptatriene, which undergoes oxidation to form tropolone. This synthesis highlights the transformation of a non-aromatic precursor into an aromatic compound.
Biological Activity[edit]
Tropolone and its derivatives have been studied for their biological activity. They exhibit antimicrobial properties and have been investigated for potential use in pharmaceuticals. The compound's ability to chelate metal ions is also of interest in the development of metal-based drugs.
Related Compounds[edit]
Tropolone is structurally related to several other compounds, including:
These compounds share the tropolone core structure and exhibit similar chemical properties.
Related Pages[edit]
References[edit]
<references group="" responsive="1"></references>
-
General synthesis of Tropolone
-
Synthesis of Tropolone from Cyclopentadiene
-
Tautomerism of Tropolone
-
Structure of Tropolone
-
Structure of Gamma-thujaplicin
-
Structure of Stipitatic acid
-
Structure of Colchicin