Nitrogen difluoride: Difference between revisions
CSV import |
CSV import Tags: mobile edit mobile web edit |
||
| Line 1: | Line 1: | ||
{{DISPLAYTITLE:Nitrogen difluoride}} | |||
== | == Nitrogen difluoride == | ||
[[File:Nitrogen_difluoride.png|thumb|right|Structural formula of nitrogen difluoride]] | |||
'''Nitrogen difluoride''' (NF<sub>2</sub>) is a chemical compound consisting of one [[nitrogen]] atom and two [[fluorine]] atoms. It is a radical species and is known for its role in various chemical reactions, particularly in the field of [[inorganic chemistry]]. | |||
== | == Structure and Properties == | ||
Nitrogen difluoride is | Nitrogen difluoride is a diatomic molecule with a bent molecular geometry. The presence of the unpaired electron on the nitrogen atom makes it a radical, which contributes to its high reactivity. The bond angle in NF<sub>2</sub> is approximately 102 degrees, and the N-F bond length is about 1.35 Å. | ||
The compound is typically found in the gas phase and is known for its instability. It can be generated through the reaction of [[nitrogen trifluoride]] (NF<sub>3</sub>) with atomic [[fluorine]] or through the photolysis of NF<sub>3</sub>. | |||
== | == Chemical Reactions == | ||
While nitrogen difluoride itself is not | Nitrogen difluoride is involved in various chemical reactions due to its radical nature. It can participate in [[radical reactions]], where it acts as an intermediate. NF<sub>2</sub> can react with other radicals or molecules, leading to the formation of more stable compounds. | ||
One notable reaction is its combination with other nitrogen-containing species to form [[nitrogen oxides]] or other nitrogen-fluorine compounds. The reactivity of NF<sub>2</sub> makes it a subject of interest in the study of [[atmospheric chemistry]] and [[combustion processes]]. | |||
== Applications == | |||
While nitrogen difluoride itself is not widely used due to its instability, its derivatives and related compounds have applications in various fields. For example, nitrogen-fluorine compounds are used in the production of [[semiconductors]] and in the [[aerospace industry]] for their high energy content. | |||
== Safety and Handling == | |||
Due to its reactive nature, nitrogen difluoride must be handled with caution. It is typically studied under controlled laboratory conditions. Proper safety protocols, including the use of [[protective equipment]] and [[ventilation]], are essential when working with NF<sub>2</sub>. | |||
== Related pages == | |||
* [[Nitrogen trifluoride]] | |||
* [[Fluorine]] | |||
* [[Radical (chemistry)]] | |||
* [[Inorganic chemistry]] | |||
[[Category:Nitrogen compounds]] | [[Category:Nitrogen compounds]] | ||
[[Category:Fluorine compounds]] | [[Category:Fluorine compounds]] | ||
[[Category:Radicals (chemistry)]] | |||
Latest revision as of 06:41, 16 February 2025
Nitrogen difluoride[edit]
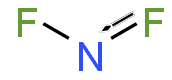
Nitrogen difluoride (NF2) is a chemical compound consisting of one nitrogen atom and two fluorine atoms. It is a radical species and is known for its role in various chemical reactions, particularly in the field of inorganic chemistry.
Structure and Properties[edit]
Nitrogen difluoride is a diatomic molecule with a bent molecular geometry. The presence of the unpaired electron on the nitrogen atom makes it a radical, which contributes to its high reactivity. The bond angle in NF2 is approximately 102 degrees, and the N-F bond length is about 1.35 Å.
The compound is typically found in the gas phase and is known for its instability. It can be generated through the reaction of nitrogen trifluoride (NF3) with atomic fluorine or through the photolysis of NF3.
Chemical Reactions[edit]
Nitrogen difluoride is involved in various chemical reactions due to its radical nature. It can participate in radical reactions, where it acts as an intermediate. NF2 can react with other radicals or molecules, leading to the formation of more stable compounds.
One notable reaction is its combination with other nitrogen-containing species to form nitrogen oxides or other nitrogen-fluorine compounds. The reactivity of NF2 makes it a subject of interest in the study of atmospheric chemistry and combustion processes.
Applications[edit]
While nitrogen difluoride itself is not widely used due to its instability, its derivatives and related compounds have applications in various fields. For example, nitrogen-fluorine compounds are used in the production of semiconductors and in the aerospace industry for their high energy content.
Safety and Handling[edit]
Due to its reactive nature, nitrogen difluoride must be handled with caution. It is typically studied under controlled laboratory conditions. Proper safety protocols, including the use of protective equipment and ventilation, are essential when working with NF2.