Birch reduction: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 20: | Line 20: | ||
{{chemistry-stub}} | {{chemistry-stub}} | ||
<gallery> | |||
File:Birch reduction general reaction.svg|Birch reduction general reaction | |||
File:Naphthalene Birch Reduction.png|Naphthalene Birch Reduction | |||
File:Birch reduction radical mechanism.svg|Birch reduction radical mechanism | |||
File:Birch R 2 startAnimGif.gif|Birch R 2 start animation | |||
File:Birch Basic-Mech.svg|Birch Basic Mechanism | |||
File:Birch-Anisole.svg|Birch Anisole | |||
File:Birch-Benzoic.svg|Birch Benzoic | |||
File:Cyclohexadienyl Anion1.svg|Cyclohexadienyl Anion | |||
File:BirchAlkylationOrgSynth1990.svg|Birch Alkylation Org Synth 1990 | |||
File:BirchAlkylation.png|Birch Alkylation | |||
</gallery> | |||
Latest revision as of 05:44, 3 March 2025

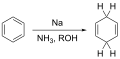
The Birch reduction is a chemical reaction that is used in organic chemistry to partially reduce aromatic rings to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch who first reported it in 1944. This reduction process involves the use of sodium, lithium, or potassium in liquid ammonia with an alcohol such as ethanol or tert-butanol as a proton source.
Mechanism[edit]
The Birch reduction mechanism involves the single-electron transfer (SET) from the metal (e.g., sodium) to the aromatic ring, forming a radical anion. This anion is then protonated by the alcohol, leading to a cyclohexadienyl radical. A second electron is transferred from another metal atom to this radical, followed by a second protonation, resulting in the formation of a 1,4-cyclohexadiene.
Applications[edit]
The Birch reduction is widely used in the synthesis of steroids, alkaloids, and other complex organic molecules. It is particularly useful for reducing aromatic rings that are sensitive to other reduction methods. The reaction allows for selective reduction, making it a valuable tool in the synthesis of various organic compounds.
Variations[edit]
Several variations of the Birch reduction exist, including modifications that allow for the reduction of pyridines and other heteroaromatic compounds. These variations often involve changes in the solvent, metal, or proton source to achieve selective reduction of specific types of aromatic rings.
Safety and Environmental Considerations[edit]
The use of liquid ammonia and reactive metals requires careful handling due to the potential for chemical burns and fire hazards. Additionally, the disposal of waste materials from the Birch reduction must be managed properly to minimize environmental impact.
-
Birch reduction general reaction
-
Naphthalene Birch Reduction
-
Birch reduction radical mechanism
-
Birch R 2 start animation
-
Birch Basic Mechanism
-
Birch Anisole
-
Birch Benzoic
-
Cyclohexadienyl Anion
-
Birch Alkylation Org Synth 1990
-
Birch Alkylation