Barium iodide: Difference between revisions
CSV import |
CSV import |
||
| Line 19: | Line 19: | ||
[[Category:Iodides]] | [[Category:Iodides]] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
<gallery> | |||
File:Cotunnite structure.png|Cotunnite structure | |||
</gallery> | |||
Latest revision as of 06:00, 3 March 2025
Barium iodide is a chemical compound with the chemical formula BaI2. It exists in anhydrous form (BaI2) as well as a dihydrate (BaI2·2H2O). Both forms are white solids at room temperature and are highly soluble in water. Barium iodide is used in various applications, including the synthesis of other barium compounds, in photography, and as a component in some types of fluorescent lamps.
Properties[edit]
Barium iodide, like other barium compounds, is toxic due to its solubility in water, which allows it to be readily absorbed by the body. The anhydrous form has a high affinity for water and can absorb moisture from the air, converting to the dihydrate form. It has a melting point of 711°C (anhydrous) and decomposes upon heating to higher temperatures, releasing iodine vapors.
Preparation[edit]
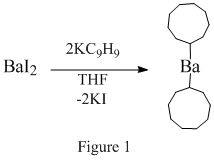
Barium iodide can be prepared by reacting barium carbonate (BaCO3) or barium sulfide (BaS) with hydroiodic acid (HI): \[BaCO_3 + 2HI \rightarrow BaI_2 + CO_2 + H_2O\] \[BaS + 2HI \rightarrow BaI_2 + H_2S\]
Applications[edit]
In the laboratory, barium iodide is used as a source of the iodide ion, a common reagent in organic synthesis. It is also employed in the production of other barium-based chemicals. In the field of photography, barium iodide has been used as a component in emulsion preparations. Additionally, its luminescent properties make it useful in the manufacturing of fluorescent lamps, where it serves as a component of the internal phosphor coating.
Safety[edit]
As with other soluble barium salts, barium iodide is toxic and poses health risks if ingested. It can cause barium poisoning, characterized by muscle weakness, cardiac irregularities, and in severe cases, paralysis or death. Handling of barium iodide should be done with appropriate safety precautions, including the use of personal protective equipment (PPE) such as gloves and safety glasses. In case of exposure, immediate medical attention is necessary.
-
Cotunnite structure
