Grotthuss mechanism: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import Tags: mobile edit mobile web edit |
||
| Line 1: | Line 1: | ||
== Grotthuss Mechanism == | |||
[[File:Proton_Zundel.gif|thumb|right|Illustration of the Grotthuss mechanism showing proton transfer in water.]] | |||
The '''Grotthuss mechanism''', also known as the '''proton hopping mechanism''', is a model that describes the movement of protons (H_ ions) through a network of hydrogen-bonded water molecules. This mechanism is fundamental to understanding the conductivity of protons in aqueous solutions and is named after Theodor Grotthuss, who proposed the concept in 1806. | |||
The | |||
== | == Mechanism Description == | ||
== | The Grotthuss mechanism involves the transfer of protons between water molecules, facilitated by the formation and breaking of [[hydrogen bonds]]. In this process, a proton is transferred from one water molecule to another, creating a chain reaction that allows for rapid proton mobility. This is distinct from the movement of other ions, which typically involves the physical movement of the ion itself through the solution. | ||
=== Proton Transfer === | |||
In the Grotthuss mechanism, a proton initially associated with a hydronium ion (H_O_) is transferred to a neighboring water molecule, converting it into a new hydronium ion. This process continues along a chain of water molecules, effectively "hopping" the proton through the network. The overall effect is a rapid movement of charge without the need for the physical displacement of the hydronium ion itself. | |||
=== Zundel and Eigen Complexes === | |||
[[File:Proton_Zundel.gif|thumb|left|Depiction of a Zundel complex, an important intermediate in the Grotthuss mechanism.]] | |||
Two important intermediates in the Grotthuss mechanism are the [[Zundel complex]] and the [[Eigen complex]]. The Zundel complex (H_O__) involves a proton shared equally between two water molecules, while the Eigen complex (H_O__) consists of a central hydronium ion surrounded by three water molecules. These complexes play a crucial role in facilitating proton transfer by stabilizing the transition states involved in the hopping process. | |||
== Importance in Chemistry == | |||
The Grotthuss mechanism is essential for understanding the high [[proton conductivity]] observed in water and other hydrogen-bonded networks. It is a key concept in fields such as [[electrochemistry]], [[biochemistry]], and [[materials science]], where proton transport is a critical factor. The mechanism also has implications for the design of [[fuel cells]] and other technologies that rely on efficient proton conduction. | |||
== Related Pages == | |||
* [[Hydrogen bond]] | * [[Hydrogen bond]] | ||
* [[Proton conductivity]] | * [[Proton conductivity]] | ||
* [[ | * [[Electrochemistry]] | ||
* [[Fuel cell]] | |||
[[Category:Physical chemistry]] | [[Category:Physical chemistry]] | ||
[[Category:Electrochemistry]] | [[Category:Electrochemistry]] | ||
Latest revision as of 11:10, 15 February 2025
Grotthuss Mechanism[edit]
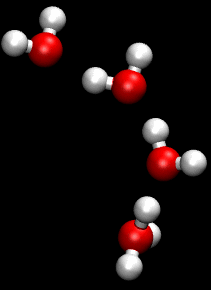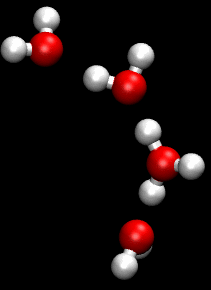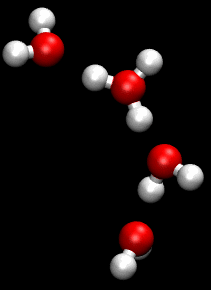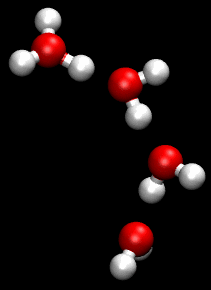
The Grotthuss mechanism, also known as the proton hopping mechanism, is a model that describes the movement of protons (H_ ions) through a network of hydrogen-bonded water molecules. This mechanism is fundamental to understanding the conductivity of protons in aqueous solutions and is named after Theodor Grotthuss, who proposed the concept in 1806.
Mechanism Description[edit]
The Grotthuss mechanism involves the transfer of protons between water molecules, facilitated by the formation and breaking of hydrogen bonds. In this process, a proton is transferred from one water molecule to another, creating a chain reaction that allows for rapid proton mobility. This is distinct from the movement of other ions, which typically involves the physical movement of the ion itself through the solution.
Proton Transfer[edit]
In the Grotthuss mechanism, a proton initially associated with a hydronium ion (H_O_) is transferred to a neighboring water molecule, converting it into a new hydronium ion. This process continues along a chain of water molecules, effectively "hopping" the proton through the network. The overall effect is a rapid movement of charge without the need for the physical displacement of the hydronium ion itself.
Zundel and Eigen Complexes[edit]

Two important intermediates in the Grotthuss mechanism are the Zundel complex and the Eigen complex. The Zundel complex (H_O__) involves a proton shared equally between two water molecules, while the Eigen complex (H_O__) consists of a central hydronium ion surrounded by three water molecules. These complexes play a crucial role in facilitating proton transfer by stabilizing the transition states involved in the hopping process.
Importance in Chemistry[edit]
The Grotthuss mechanism is essential for understanding the high proton conductivity observed in water and other hydrogen-bonded networks. It is a key concept in fields such as electrochemistry, biochemistry, and materials science, where proton transport is a critical factor. The mechanism also has implications for the design of fuel cells and other technologies that rely on efficient proton conduction.