Smiles rearrangement: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 30: | Line 30: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
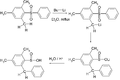
File:Smiles_rearrangement.svg|Smiles rearrangement | |||
File:Truce_Smiles_rearrangement.png|Truce-Smile rearrangement | |||
</gallery> | |||
Latest revision as of 01:00, 18 February 2025
Smiles Rearrangement is a chemical reaction that involves the intramolecular migration of an atom or group of atoms from one position to another within a molecule. This rearrangement is named after the British chemist Samuel Smiles who first reported it. The Smiles rearrangement is a significant reaction in organic chemistry, particularly in the synthesis and modification of complex organic molecules. It is characterized by its ability to efficiently alter the structure of aromatic compounds, making it a valuable tool in the development of pharmaceuticals, agrochemicals, and other organic materials.
Mechanism[edit]
The mechanism of the Smiles rearrangement involves the nucleophilic attack of an aromatic or heteroaromatic system by a leaving group, typically a sulfonate, which is located in the molecule. This attack leads to the formation of a new sigma bond and the migration of the substituent from one atom to another within the molecule. The process is facilitated by the presence of a good leaving group and is often catalyzed by bases or acids, depending on the specific substrates and conditions.
Types of Smiles Rearrangement[edit]
There are several variations of the Smiles rearrangement, each defined by the nature of the migrating group and the conditions under which the reaction occurs. These include:
- Thermal Smiles Rearrangement: Occurs under high temperature without the need for a catalyst.
- Photochemical Smiles Rearrangement: Initiated by the absorption of light, leading to the migration of groups within a molecule.
- Catalytic Smiles Rearrangement: Involves the use of a catalyst, typically a base or an acid, to facilitate the migration process.
Applications[edit]
The Smiles rearrangement has found widespread application in organic synthesis. It is particularly useful in the construction of complex molecular architectures, including natural products and biologically active molecules. The ability to rearrange atoms within a molecule allows chemists to efficiently synthesize compounds that would otherwise be difficult to obtain through conventional methods.
Limitations[edit]
Despite its utility, the Smiles rearrangement has some limitations. The reaction conditions can sometimes be harsh, and the selectivity of the migration can be difficult to control. Additionally, the reaction may not proceed efficiently for certain substrates, limiting its applicability in some cases.
See Also[edit]
References[edit]
<references/>
-
Smiles rearrangement
-
Truce-Smile rearrangement