Tetramethylsilane: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import Tags: mobile edit mobile web edit |
||
| Line 1: | Line 1: | ||
{{DISPLAYTITLE:Tetramethylsilane}} | |||
== | == Tetramethylsilane == | ||
[[Tetramethylsilane]] | [[File:Tetramethylsilane_2D_flat.svg|thumb|right|200px|Structural formula of Tetramethylsilane]] | ||
'''Tetramethylsilane''' (TMS) is an organosilicon compound with the formula (CH_)_Si. It is the simplest [[silane]] with four [[methyl group|methyl groups]] attached to a central [[silicon]] atom. TMS is a colorless, volatile liquid at room temperature and is notable for its use as a reference standard in [[nuclear magnetic resonance]] (NMR) spectroscopy. | |||
Tetramethylsilane is | |||
Tetramethylsilane | == Structure and Properties == | ||
Tetramethylsilane is a [[tetrahedral]] molecule, with the silicon atom at the center and four methyl groups symmetrically arranged around it. This symmetry makes TMS a non-polar molecule, contributing to its low boiling point of 26.5 °C. The molecule's lack of polarity and low molecular weight make it an ideal reference compound in NMR spectroscopy. | |||
== | == Uses == | ||
The primary use of tetramethylsilane is as a reference standard in [[proton nuclear magnetic resonance|_H NMR]] and [[carbon-13 nuclear magnetic resonance|__C NMR]] spectroscopy. In NMR, the chemical shift of TMS is defined as 0 ppm, providing a baseline for measuring the chemical shifts of other compounds. This is due to its single, sharp peak in the NMR spectrum, which arises from the equivalent environment of all hydrogen atoms in the molecule. | |||
== Safety == | == Safety and Handling == | ||
Tetramethylsilane is a | Tetramethylsilane is a flammable liquid and should be handled with care. It should be stored in a cool, well-ventilated area away from sources of ignition. In case of contact with skin or eyes, it is important to rinse thoroughly with water and seek medical attention if necessary. | ||
== | == Related Compounds == | ||
TMS is part of a broader class of organosilicon compounds, which include other [[alkylsilane]]s and [[silicone]]s. These compounds are widely used in various industrial applications, including as [[silicone rubber|silicone rubbers]], [[sealant]]s, and [[lubricant]]s. | |||
== Related Pages == | |||
* [[Silane]] | * [[Silane]] | ||
* [[ | * [[Nuclear magnetic resonance spectroscopy]] | ||
* [[ | * [[Organosilicon chemistry]] | ||
[[Category:Organosilicon compounds]] | [[Category:Organosilicon compounds]] | ||
[[Category: | [[Category:Nuclear magnetic resonance]] | ||
Latest revision as of 05:44, 16 February 2025
Tetramethylsilane[edit]
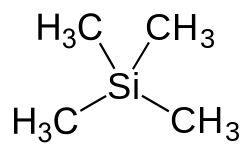
Tetramethylsilane (TMS) is an organosilicon compound with the formula (CH_)_Si. It is the simplest silane with four methyl groups attached to a central silicon atom. TMS is a colorless, volatile liquid at room temperature and is notable for its use as a reference standard in nuclear magnetic resonance (NMR) spectroscopy.
Structure and Properties[edit]
Tetramethylsilane is a tetrahedral molecule, with the silicon atom at the center and four methyl groups symmetrically arranged around it. This symmetry makes TMS a non-polar molecule, contributing to its low boiling point of 26.5 °C. The molecule's lack of polarity and low molecular weight make it an ideal reference compound in NMR spectroscopy.
Uses[edit]
The primary use of tetramethylsilane is as a reference standard in _H NMR and __C NMR spectroscopy. In NMR, the chemical shift of TMS is defined as 0 ppm, providing a baseline for measuring the chemical shifts of other compounds. This is due to its single, sharp peak in the NMR spectrum, which arises from the equivalent environment of all hydrogen atoms in the molecule.
Safety and Handling[edit]
Tetramethylsilane is a flammable liquid and should be handled with care. It should be stored in a cool, well-ventilated area away from sources of ignition. In case of contact with skin or eyes, it is important to rinse thoroughly with water and seek medical attention if necessary.
Related Compounds[edit]
TMS is part of a broader class of organosilicon compounds, which include other alkylsilanes and silicones. These compounds are widely used in various industrial applications, including as silicone rubbers, sealants, and lubricants.